Analytical Evaluation of Point-of-Care Finecare™ Procalcitonin Rapid Quantitative Test in Sepsis Population as Compared with Elecsys® BRAHMS Procalcitonin Immunoassay
- PMID: 38893607
- PMCID: PMC11172286
- DOI: 10.3390/diagnostics14111080
Analytical Evaluation of Point-of-Care Finecare™ Procalcitonin Rapid Quantitative Test in Sepsis Population as Compared with Elecsys® BRAHMS Procalcitonin Immunoassay
Abstract
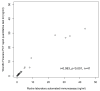
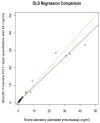
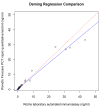
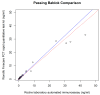
The study compared two plasma procalcitonin (PCT) assays, the point of care (POC) Finecare™ Procalcitonin Rapid Quantitative Test and the Elecsys® BRAHMS PCT immunoassay, in sepsis ICU patients. Forty-one plasma samples were analyzed, showing a strong correlation (r = 0.98) and no significant difference in PCT values. The mean POC PCT value was 4.46 ng/mL (SD 8.68), and for laboratory BRAHMS PCT, it was 4.67 ng/mL (SD 10.03). The study found a strong linear relationship between plasma POC PCT and laboratory BRAHMS PCT (r = 0.98). Different regression methods showed varying intercepts and slopes: Ordinary Least Squares had an intercept of 0.49 and a slope of 0.85; Deming regression showed an intercept of 0.43 and a slope of 0.86; Passing-Bablok regression showed an intercept of 0.02 and a slope of 1.08. Precision results for cut-offs of 0.5 ng/mL were a coefficient of variation (CV) of 5%, and for 2.5 ng/mL, the CV was 2.5%. The Pearson correlation coefficient (r) for linearity was ≥0.99. The study revealed no significant difference between the POC Finecare™ PCT and Elecsys® BRAHMS PCT immunoassay in sepsis samples from ICU patients, supported by strong correlation, minimal bias, a consistent CV, and linearity.
Keywords: intensive care unit; point of care; procalcitonin; sepsis.
Conflict of interest statement
The funding sources did not influence the study’s design, data collection, analysis, interpretation, manuscript writing, or decision to publish.
Figures
Similar articles
-
Analytical evaluation of the novel Lumipulse G BRAHMS procalcitonin immunoassay.Pract Lab Med. 2016 Jul 16;6:8-13. doi: 10.1016/j.plabm.2016.07.001. eCollection 2016 Dec 1. Pract Lab Med. 2016. PMID: 28856208 Free PMC article.
-
Analytical and clinical performance of the Advansure i3 procalcitonin assay.Scand J Clin Lab Invest. 2021 Nov;81(7):546-551. doi: 10.1080/00365513.2021.1969592. Epub 2021 Oct 4. Scand J Clin Lab Invest. 2021. PMID: 34601986
-
Comparison of the Abbott Architect BRAHMS and the Biomérieux Vidas BRAHMS Procalcitonin Assays.J Appl Lab Med. 2019 Jan;3(4):580-586. doi: 10.1373/jalm.2018.027268. Epub 2018 Sep 19. J Appl Lab Med. 2019. PMID: 31639727
-
The use of procalcitonin for the management of sepsis in Internal Medicine wards: current evidence.Panminerva Med. 2020 Mar;62(1):54-62. doi: 10.23736/S0031-0808.19.03809-6. Epub 2019 Nov 11. Panminerva Med. 2020. PMID: 31729202 Review.
-
Progress in Procalcitonin Detection Based on Immunoassay.Research (Wash D C). 2024 Apr 16;7:0345. doi: 10.34133/research.0345. eCollection 2024. Research (Wash D C). 2024. PMID: 38711476 Free PMC article. Review.
References
-
- Bouadma L., Luyt C.-E., Tubach F., Cracco C., Alvarez A., Schwebel C., Schortgen F., Lasocki S., Veber B., Dehoux M., et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): A multicentre randomised controlled trial. Lancet. 2010;375:463–474. doi: 10.1016/S0140-6736(09)61879-1. - DOI - PubMed
-
- Evans L., Rhodes A., Alhazzani W., Antonelli M., Coopersmith C.M., French C., Machado F.R., Mcintyre L., Ostermann M., Prescott H.C., et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Crit. Care Med. 2021;49:E1063–E1143. doi: 10.1097/CCM.0000000000005337. - DOI - PubMed
LinkOut - more resources
Full Text Sources