Filtered Saliva for Rapid and Accurate Analyte Detection for POC Diagnostics
- PMID: 38893615
- PMCID: PMC11171550
- DOI: 10.3390/diagnostics14111088
Filtered Saliva for Rapid and Accurate Analyte Detection for POC Diagnostics
Abstract
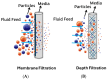
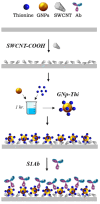
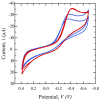
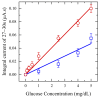
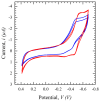
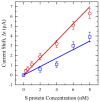
Saliva has shown considerable promise as a diagnostic medium for point-of-care (POC) and over-the-counter (OTC) diagnostic devices due to the non-invasive nature of its collection. However, a significant limitation of saliva-based detection is undesirable interference in a sensor's readout caused by interfering components in saliva. In this study, we develop standardized sample treatment procedures to eliminate bubbles and interfering molecules while preserving the sample's target molecules such as spike (S) protein and glucose. We then test the compatibility of the pretreatment system with our previously designed SARS-CoV-2 and glucose diagnostic biosensing systems for detecting S protein and glucose in subject saliva. Ultimately, the effectiveness of each filter in enhancing biomarker sensitivity is assessed. The results show that a 20 mg nylon wool (NW) filter shows an 80% change in viscosity reduction with only a 6% reduction in protein content, making it an appropriate filter for the salivary S protein diagnostic system. Meanwhile, a 30 mg cotton wool (CW) filter is identified as the optimal choice for salivary glucose detection, achieving a 90% change in viscosity reduction and a 60.7% reduction in protein content with a minimal 4.3% reduction in glucose content. The NW pretreatment filtration significantly improves the limit of detection (LOD) for salivary S protein detection by five times (from 0.5 nM to 0.1 nM) and it reduces the relative standard deviation (RSD) two times compared to unfiltered saliva. Conversely, the CW filter used for salivary glucose detection demonstrated improved linearity with an R2 of 0.99 and a sensitivity of 36.6 μA/mM·cm2, over twice as high as unfiltered saliva. This unique filtration process can be extended to any POC diagnostic system and optimized for any biomarker detection, making electrochemical POC diagnostics more viable in the current market.
Keywords: SARS-CoV-2 spike protein; diagnostics; electrochemical biosensor; glucose; point of care (POC) devices; saliva; saliva filtration process.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
A Rapid Label-Free Disposable Electrochemical Salivary Point-of-Care Sensor for SARS-CoV-2 Detection and Quantification.Sensors (Basel). 2022 Dec 30;23(1):433. doi: 10.3390/s23010433. Sensors (Basel). 2022. PMID: 36617031 Free PMC article.
-
Lab-on-a-Disc for Point-of-Care Infection Diagnostics.Acc Chem Res. 2021 Oct 5;54(19):3643-3655. doi: 10.1021/acs.accounts.1c00367. Epub 2021 Sep 13. Acc Chem Res. 2021. PMID: 34516092
-
Saliva-based microfluidic point-of-care diagnostic.Theranostics. 2023 Jan 31;13(3):1091-1108. doi: 10.7150/thno.78872. eCollection 2023. Theranostics. 2023. PMID: 36793864 Free PMC article. Review.
-
Point-of-care platforms for salivary diagnostics.Chin J Dent Res. 2012;15(1):7-15. Chin J Dent Res. 2012. PMID: 22866276
-
A Review on Saliva-Based Health Diagnostics: Biomarker Selection and Future Directions.Biomed Mater Devices. 2023 Jun 6:1-18. doi: 10.1007/s44174-023-00090-z. Online ahead of print. Biomed Mater Devices. 2023. PMID: 37363139 Free PMC article. Review.
Cited by
-
Measurement of Salivary Cortisol for Revealing Age-Specific Dependence of Cortisol Levels on Time, Feeding, and Oxygen Metabolism in Newborn Infants.Biosensors (Basel). 2025 Jul 1;15(7):420. doi: 10.3390/bios15070420. Biosensors (Basel). 2025. PMID: 40710070 Free PMC article.
-
AuNP/Magnetic Bead-Enhanced Electrochemical Sensor Toward Dual Saliva Alzheimer's Biomarkers Detection.Sensors (Basel). 2025 Jun 30;25(13):4088. doi: 10.3390/s25134088. Sensors (Basel). 2025. PMID: 40648343 Free PMC article.
References
-
- Florkowski C., Don-Wauchope A., Gimenez N., Rodriguez-Capote K., Wils J., Zemlin A. Point-of-care testing (POCT) and evidence-based laboratory medicine (EBLM)—Does it leverage any advantage in clinical decision making? Crit. Rev. Clin. Lab. Sci. 2017;54:471–494. doi: 10.1080/10408363.2017.1399336. - DOI - PubMed
-
- Esfahani I.C., Sun H. A droplet-based micropillar-enhanced acoustic wave (μPAW) device for viscosity measurement. Sens. Actuators A Phys. 2023;350:114121. doi: 10.1016/j.sna.2022.114121. - DOI
-
- Da Silva E.T.S.G., Souto D.E.P., Barragan J.T.C., Giarola J.d.F., de Moraes A.C.M., Kubota L.T. Electrochemical biosensors in point-of-care devices: Recent advances and future trends. ChemElectroChem. 2017;4:778–794. doi: 10.1002/celc.201600758. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

