Developing a Novel Murine Meningococcal Meningitis Model Using a Capsule-Null Bacterial Strain
- PMID: 38893642
- PMCID: PMC11172168
- DOI: 10.3390/diagnostics14111116
Developing a Novel Murine Meningococcal Meningitis Model Using a Capsule-Null Bacterial Strain
Abstract
Background: Neisseria meningitidis (meningococcus) is a Gram-negative bacterium that colonises the nasopharynx of about 10% of the healthy human population. Under certain conditions, it spreads into the body, causing infections with high morbidity and mortality rates. Although the capsule is the key virulence factor, unencapsulated strains have proved to possess significant clinical implications as well. Meningococcal meningitis is a primarily human infection, with limited animal models that are dependent on a variety of parameters such as bacterial virulence and mouse strain. In this study, we aimed to develop a murine Neisseria meningitidis meningitis model to be used in the study of various antimicrobial compounds.
Method: We used a capsule-deficient Neisseria meningitidis strain that was thoroughly analysed through various methods. The bacterial strain was incubated for 48 h in brain-heart infusion (BHI) broth before being concentrated and injected intracisternally to bypass the blood-brain barrier in CD-1 mice. This prolonged incubation time was a key factor in increasing the virulence of the bacterial strain. A total of three more differently prepared inoculums were tested to further solidify the importance of the protocol (a 24-h incubated inoculum, a diluted inoculum, and an inactivated inoculum). Antibiotic treatment groups were also established. The clinical parameters and number of deaths were recorded over a period of 5 days, and comatose mice with no chance of recovery were euthanised.
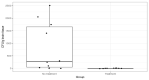
Results: The bacterial strain was confirmed to have no capsule but was found to harbour a total of 56 genes coding virulence factors, and its antibiotic susceptibility was established. Meningitis was confirmed through positive tissue culture and histological evaluation, where specific lesions were observed, such as perivascular sheaths with inflammatory infiltrate. In the treatment groups, survival rates were significantly higher (up to 81.25% in one of the treatment groups compared to 18.75% in the control group).
Conclusion: We managed to successfully develop a cost-efficient murine (using simple CD-1 mice instead of expensive transgenic mice) meningococcal meningitis model using an unencapsulated strain with a novel method of preparation.
Keywords: Neisseria meningitidis; capsule deficient meningococcus; capsule null locus; meningococcal meningitis; murine model.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Meningococcus—PAHO/WHO|Pan American Health Organization. [(accessed on 10 March 2024)]. Available online: https://www.paho.org/en/topics/meningococcus.
-
- Joshi R., Saroj S.D. Survival and Evasion of Neisseria meningitidis from Macrophages. Med. Microecol. 2023;17:100087. doi: 10.1016/j.medmic.2023.100087. - DOI
LinkOut - more resources
Full Text Sources

