Core-Double-Shell TiO2@Fe3O4@C Microspheres with Enhanced Cycling Performance as Anode Materials for Lithium-Ion Batteries
- PMID: 38893808
- PMCID: PMC11173600
- DOI: 10.3390/ma17112543
Core-Double-Shell TiO2@Fe3O4@C Microspheres with Enhanced Cycling Performance as Anode Materials for Lithium-Ion Batteries
Abstract
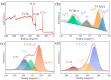
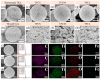
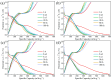
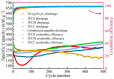
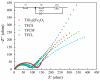
Due to the volume expansion effect during charge and discharge processes, the application of transition metal oxide anode materials in lithium-ion batteries is limited. Composite materials and carbon coating are often considered feasible improvement methods. In this study, three types of TiO2@Fe3O4@C microspheres with a core-double-shell structure, namely TFCS (TiO2@Fe3O4@C with 0.0119 g PVP), TFCM (TiO2@Fe3O4@C with 0.0238 g PVP), and TFCL (TiO2@Fe3O4@C with 0.0476 g PVP), were prepared using PVP (polyvinylpyrrolidone) as the carbon source through homogeneous precipitation and high-temperature carbonization methods. After 500 cycles at a current density of 2 C, the specific capacities of these three microspheres are all higher than that of TiO2@Fe2O3 with significantly improved cycling stability. Among them, TFCM exhibits the highest specific capacity of 328.3 mAh·g-1, which was attributed to the amorphous carbon layer effectively mitigating the capacity decay caused by the volume expansion of iron oxide during charge and discharge processes. Additionally, the carbon coating layer enhances the electrical conductivity of the TiO2@Fe3O4@C materials, thereby improving their rate performance. Within the range of 100 to 1600 mA·g-1, the capacity retention rates for TiO2@Fe2O3, TFCS, TFCM, and TFCL are 27.2%, 35.2%, 35.9%, and 36.9%, respectively. This study provides insights into the development of new lithium-ion battery anode materials based on Ti and Fe oxides with the abundance and environmental friendliness of iron, titanium, and carbon resources in TiO2@Fe3O4@C microsphere anode materials, making this strategy potentially applicable.
Keywords: anode materials; carbon coating; core–double-shell structure; electrochemical properties; lithium-ion batteries.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Lychee-like TiO2@Fe2O3 Core-Shell Nanostructures with Improved Lithium Storage Properties as Anode Materials for Lithium-Ion Batteries.Materials (Basel). 2023 Feb 27;16(5):1945. doi: 10.3390/ma16051945. Materials (Basel). 2023. PMID: 36903060 Free PMC article.
-
Bio-Inspired Hierarchical Nanofibrous Fe3O4-TiO2-Carbon Composite as a High-Performance Anode Material for Lithium-Ion Batteries.ACS Appl Mater Interfaces. 2016 Jul 13;8(27):17343-51. doi: 10.1021/acsami.6b05206. Epub 2016 Jun 29. ACS Appl Mater Interfaces. 2016. PMID: 27328774
-
Toward Highly Stable Anode for Secondary Batteries: Employing TiO2 Shell as Elastic Buffering Marix for FeOx Nanoparticles.Small. 2022 Mar;18(11):e2105713. doi: 10.1002/smll.202105713. Epub 2022 Jan 20. Small. 2022. PMID: 35060316
-
Titanium dioxide-based anode materials for lithium-ion batteries: structure and synthesis.RSC Adv. 2022 Nov 23;12(52):33641-33652. doi: 10.1039/d2ra05442f. eCollection 2022 Nov 22. RSC Adv. 2022. PMID: 36505712 Free PMC article. Review.
-
Recent Advances in the Application of Magnetite (Fe3O4) in Lithium-Ion Batteries: Synthesis, Electrochemical Performance, and Characterization Techniques.Chem Mater. 2024 Sep 18;36(19):9299-9319. doi: 10.1021/acs.chemmater.4c02013. eCollection 2024 Oct 8. Chem Mater. 2024. PMID: 39398366 Free PMC article. Review.
Cited by
-
Rapid Synthesis of Fast-Charging TiNb2O7 for Lithium-Ion Storage via Ultrafast Carbothermal Shock.Micromachines (Basel). 2025 Apr 22;16(5):490. doi: 10.3390/mi16050490. Micromachines (Basel). 2025. PMID: 40428617 Free PMC article.
References
-
- Shetti N.P., Dias S., Reddy K.R. Nanostructured Organic and Inorganic Materials for Li-Ion Batteries: A Review. Mater. Sci. Semicond. Process. 2019;104:104684. doi: 10.1016/j.mssp.2019.104684. - DOI
-
- Kim J., Hwang J., Sun Y., Hassoun J. A Single Layer of Fe3O4@TiO2 Submicron Spheres as a High-Performance Electrode for Lithium-Ion Microbatteries. Sustain. Energy Fuels. 2019;3:2675–2687. doi: 10.1039/C9SE00259F. - DOI
-
- Li Z., Fu N., Yang Z. Particulate Modification of Lithium-Ion Battery Anode Materials and Electrolytes. Particuology. 2023;83:129–141. doi: 10.1016/j.partic.2023.02.010. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

