Oligoester Identification in the Inner Coatings of Metallic Cans by High-Pressure Liquid Chromatography-Mass Spectrometry with Cone Voltage-Induced Fragmentation
- PMID: 38894033
- PMCID: PMC11173705
- DOI: 10.3390/ma17112771
Oligoester Identification in the Inner Coatings of Metallic Cans by High-Pressure Liquid Chromatography-Mass Spectrometry with Cone Voltage-Induced Fragmentation
Abstract
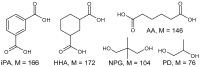
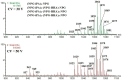
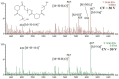
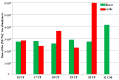
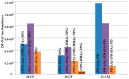
The application of polyesters as food contact materials is an alternative to epoxy resin coatings, which can be a source of endocrine migrants. By using high-pressure liquid chromatography/electrospray ionization-mass spectrometry (HPLC/ESI-MS) with cone voltage-induced fragmentation in-source, a number of polyester-derived migrants were detected in the extracts of inner coatings of metallic cans. The polyester-derived migrants were detected in each inner coating of fish product-containing cans (5/5) and in one inner coating of meat product-containing can (1/5). They were not detected in the inner coatings of vegetable/fruit product-containing cans (10 samples). The respective detected parent and product ions enabled differentiation between cyclic and linear compounds, as well as unambiguous identification of diol and diacid units. Most of the detected compounds, cyclic and linear, were composed of neopentyl glycol as diol and two diacid comonomers, namely isophthalic acid and hexahydrophthalic acid. The other detected oligoesters were composed of neopentyl glycol or propylene glycol and adipic acid/isophthalic acid as comonomers. The compounds containing propylene glycol as diol were found to be exclusively linear cooligoesters. On the basis of abundances of [M+Na]+ ions, the relative contents of cyclic and linear oligoesters were evaluated.
Keywords: can-coating material; fragmentation pathway; liquid chromatography; mass spectrometry; polyesters.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
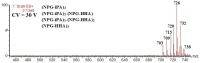
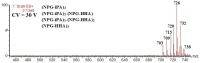
References
-
- Abdollahi M., Khalili B. Development of a novel hyperbranched unsaturated polyester resin: Synthesis, characterization, and potential applications in car body putty. Curr. Chem. Lett. 2024;13:325–334. doi: 10.5267/j.ccl.2023.11.006. - DOI
-
- Pellis A., Herrero Acero E., Gardossi L., Ferrario V., Guebitz G.M. Renewable building blocks for sustainable polyesters: New biotechnological routes for greener plastics. Polym. Int. 2016;65:861–871. doi: 10.1002/pi.5087. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

