Point-of-Care Fluorescence Biosensing System for Rapid Multi-Allergen Screening
- PMID: 38894075
- PMCID: PMC11174465
- DOI: 10.3390/s24113280
Point-of-Care Fluorescence Biosensing System for Rapid Multi-Allergen Screening
Abstract
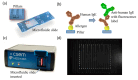
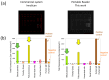
With the steady increase in allergy prevalence worldwide, there is a strong need for novel diagnostic tools for precise, fast, and less invasive testing methods. Herein, a miniatured fluorescence-based biosensing system is developed for the rapid and quantitative detection of allergen-specific immunoglobulin-E. An antibody-based fluorescence assay in a microfluidic-patterned slide, combined with a custom-made portable fluorescence reader for image acquisition and user-friendly software for the data analysis, enables obtaining results for multiple allergens in just ~1 h with only 80 μL of blood serum. The multiplexed detection of common birch, timothy grass, cat epithelia, house dust mite, and dog epithelia shows quantitative IgE-mediated allergic responses to specific allergens in control serum samples with known total IgE concentration. The responses are verified with different control tests and measurements with a commercial fluorescence reader. These results open the door to point-of-care allergy screening for early diagnosis and broader access and for large-scale research in allergies.
Keywords: IgE; allergen; allergy; antibody; biosensor; fluorescence; microfluidics; optical reader; point of care; portable reader.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Laiho J.E., Laitinen O.H., Malkamäki J., Puustinen L., Sinkkonen A., Pärkkä J., Hyöty H., Anna E., Heikki H., Kalle K., et al. Exposomic Determinants of Immune-Mediated Diseases: Special Focus on Type 1 Diabetes, Celiac Disease, Asthma, and Allergies: The HEDIMED Project Approach. Environ. Epidemiol. 2022;6:e212. doi: 10.1097/EE9.0000000000000212. - DOI - PMC - PubMed
-
- World Allergy Organization . In: White Book on Allergy. 2011th ed. Pawankar R., Canonica G.W., Holgate S.T., Lockey R.F., editors. World Allergy Organization (WAO); London, UK: 2011. pp. 1–225.
-
- Nowak-Węgrzyn A., Assa’ad A.H., Bahna S.L., Bock S.A., Sicherer S.H., Teuber S.S., Adverse Reactions to Food Committee of the American Academy of Allergy, Asthma & Immunology Work Group Report: Oral Food Challenge Testing. J. Allergy Clin. Immunol. 2009;123:S365–S378. doi: 10.1016/j.jaci.2009.03.042. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

