Construction of Chitosan-Modified Naphthalimide Fluorescence Probe for Selective Detection of Cu2
- PMID: 38894218
- PMCID: PMC11174907
- DOI: 10.3390/s24113425
Construction of Chitosan-Modified Naphthalimide Fluorescence Probe for Selective Detection of Cu2
Abstract
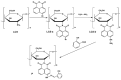
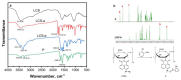
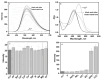
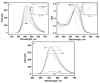
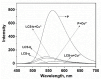
A chitosan-based Cu2+ fluorescent probe was designed and synthesized independently using the C-2-amino group of chitosan with 1, 8-naphthalimide derivatives. A series of experiments were conducted to characterize the optical properties of the grafted probe. The fluorescence quenching effect was investigated based on the interactions between the probe and common metals. It was found that the proposed probe displayed selective interaction with Cu2+ over other metal ions and anions, reaching equilibrium within 5 min.
Keywords: 1, 8-naphthalimide; Cu2+; chitosan; fluorescence.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Synthesis and Application of 1,8-Naphthalimide Derivatives Fluorescent Probe for Sequential Recognition of Cu2+ and H2PO4.J Fluoresc. 2025 May;35(5):2685-2694. doi: 10.1007/s10895-024-03692-y. Epub 2024 Apr 13. J Fluoresc. 2025. PMID: 38613712
-
A retrievable and highly selective fluorescent probe for monitoring dihydrogen phosphate ions based on a naphthalimide framework.Spectrochim Acta A Mol Biomol Spectrosc. 2013 Oct;114:323-9. doi: 10.1016/j.saa.2013.05.072. Epub 2013 May 30. Spectrochim Acta A Mol Biomol Spectrosc. 2013. PMID: 23786971
-
The mechanism of fluorescence quenching of naphthalimide A/C leak detector by copper (II).BMC Chem. 2023 Jul 5;17(1):69. doi: 10.1186/s13065-023-00987-2. BMC Chem. 2023. PMID: 37407990 Free PMC article.
-
A naphthalimide functionalized chitosan-based fluorescent probe for specific detection and efficient adsorption of Cu2.Int J Biol Macromol. 2023 Jun 1;239:124261. doi: 10.1016/j.ijbiomac.2023.124261. Epub 2023 Mar 30. Int J Biol Macromol. 2023. PMID: 37003383
-
A Novel Rhodamine B Fluorescent Probe Derived from Carboxymethyl Chitosan for the Selective Detection of Fe3.Polymers (Basel). 2024 Nov 19;16(22):3206. doi: 10.3390/polym16223206. Polymers (Basel). 2024. PMID: 39599296 Free PMC article.
References
-
- Han T., Yuan Y., Kang H., Zhang Y., Dong L. Ultrafast, sensitive and visual sensing of copper ions by a dual-fluorescent film based on quantum dots. J. Mater. Chem. C. 2019;7:14904–14912. doi: 10.1039/C9TC04592A. - DOI
-
- Gupta A.S., Paul K., Luxami V. A fluorescent probe with “AIE+ESIPT” characteristics for Cu2+ and F− ions estimation. Sens. Actuators B Chem. 2017;246:653–661. doi: 10.1016/j.snb.2017.02.080. - DOI
-
- Erdemir S., Malkondu S., Kocyigit O. A blue/red dual-emitting multi-responsive fluorescent probe for Fe3+, Cu2+ and cysteine based on isophorone-antharecene. Microchem. J. 2020;157:105075. doi: 10.1016/j.microc.2020.105075. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

