AKT Inhibition Sensitizes to Polo-Like Kinase 1 Inhibitor Onvansertib in Prostate Cancer
- PMID: 38894678
- PMCID: PMC11444904
- DOI: 10.1158/1535-7163.MCT-23-0933
AKT Inhibition Sensitizes to Polo-Like Kinase 1 Inhibitor Onvansertib in Prostate Cancer
Abstract
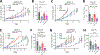
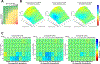
Polo-like kinase 1 (PLK1) inhibitors have had limited antitumor efficacy as single agents, and focus of current efforts is on combination therapies. We initially confirmed that the PLK1-specific inhibitor onvansertib (ONV) could enhance responses to a PARP inhibitor (olaparib) in prostate cancer xenografts. To identify more effective combinations, we screened a library of bioactive compounds for efficacy in combination with ONV in LNCaP prostate cancer cells, which identified a series of compounds including multiple AKT inhibitors. We confirmed in vitro synergy between ONV and the AKT inhibitor ipatasertib (IPA) and found that the combination increased apoptosis. Mechanistic studies showed that ONV increased expression of the antiapoptotic protein SURVIVIN and that this was mitigated by IPA. Studies in three PTEN-deficient prostate cancer xenograft models showed that cotreatment with IPA and ONV led to significant tumor growth inhibition compared with monotherapies. Together, these in vitro and in vivo studies demonstrate that the efficacy of PLK1 antagonists can be enhanced by PARP or AKT inhibition and support further development of these combination therapies.
©2024 American Association for Cancer Research.
Conflict of interest statement
Figures
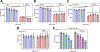




Similar articles
-
Synergistic two-step inhibition approach using a combination of trametinib and onvansertib in KRAS and TP53-mutated colorectal adenocarcinoma.Biomed Pharmacother. 2025 Jan;182:117796. doi: 10.1016/j.biopha.2024.117796. Epub 2024 Dec 28. Biomed Pharmacother. 2025. PMID: 39731938
-
Augmented antitumor activity by olaparib plus AZD1775 in gastric cancer through disrupting DNA damage repair pathways and DNA damage checkpoint.J Exp Clin Cancer Res. 2018 Jun 28;37(1):129. doi: 10.1186/s13046-018-0790-7. J Exp Clin Cancer Res. 2018. PMID: 29954437 Free PMC article.
-
Targeting Plk1 to Enhance Efficacy of Olaparib in Castration-Resistant Prostate Cancer.Mol Cancer Ther. 2017 Mar;16(3):469-479. doi: 10.1158/1535-7163.MCT-16-0361. Epub 2017 Jan 9. Mol Cancer Ther. 2017. PMID: 28069876 Free PMC article.
-
BI_2536--targeting the mitotic kinase Polo-like kinase 1 (Plk1).Recent Results Cancer Res. 2010;184:215-8. doi: 10.1007/978-3-642-01222-8_15. Recent Results Cancer Res. 2010. PMID: 20072841 Review.
-
Modulating polo-like kinase 1 as a means for cancer chemoprevention.Pharm Res. 2010 Jun;27(6):989-98. doi: 10.1007/s11095-010-0051-8. Epub 2010 Jan 27. Pharm Res. 2010. PMID: 20107874 Free PMC article. Review.
Cited by
-
Onvansertib exhibits anti-proliferative and anti-invasive effects in endometrial cancer.Front Pharmacol. 2025 Mar 17;16:1545038. doi: 10.3389/fphar.2025.1545038. eCollection 2025. Front Pharmacol. 2025. PMID: 40166466 Free PMC article.
-
PLK1 Inhibitor Onvansertib Enhances the Efficacy of Alpelisib in PIK3CA-Mutated HR-Positive Breast Cancer Resistant to Palbociclib and Endocrine Therapy: Preclinical Insights.Cancers (Basel). 2024 Sep 25;16(19):3259. doi: 10.3390/cancers16193259. Cancers (Basel). 2024. PMID: 39409880 Free PMC article.
-
Aurora kinases signaling in cancer: from molecular perception to targeted therapies.Mol Cancer. 2025 Jun 18;24(1):180. doi: 10.1186/s12943-025-02353-3. Mol Cancer. 2025. PMID: 40533769 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

