Distinct DNA repair mechanisms prevent formaldehyde toxicity during development, reproduction and aging
- PMID: 38894680
- PMCID: PMC11317141
- DOI: 10.1093/nar/gkae519
Distinct DNA repair mechanisms prevent formaldehyde toxicity during development, reproduction and aging
Abstract
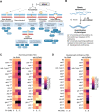
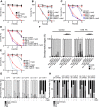
Formaldehyde (FA) is a recognized environmental and metabolic toxin implicated in cancer development and aging. Inherited mutations in the FA-detoxifying enzymes ADH5 and ALDH2 genes lead to FA overload in the severe multisystem AMeD syndrome. FA accumulation causes genome damage including DNA-protein-, inter- and intra-strand crosslinks and oxidative lesions. However, the influence of distinct DNA repair systems on organismal FA resistance remains elusive. We have here investigated the consequence of a range of DNA repair mutants in a model of endogenous FA overload generated by downregulating the orthologs of human ADH5 and ALDH2 in C. elegans. We have focused on the distinct components of nucleotide excision repair (NER) during developmental growth, reproduction and aging. Our results reveal three distinct modes of repair of FA-induced DNA damage: Transcription-coupled repair (TCR) operating NER-independently during developmental growth or through NER during adulthood, and, in concert with global-genome (GG-) NER, in the germline and early embryonic development. Additionally, we show that the Cockayne syndrome B (CSB) factor is involved in the resolution of FA-induced DNA-protein crosslinks, and that the antioxidant and FA quencher N-acetyl-l-cysteine (NAC) reverses the sensitivity of detoxification and DNA repair defects during development, suggesting a therapeutic intervention to revert FA-pathogenic consequences.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

