A Multicenter Phase 2 Trial Evaluating the Efficacy and Safety of Preoperative Lenvatinib Therapy for Patients with Advanced Hepatocellular Carcinoma (LENS-HCC Trial)
- PMID: 38894811
- PMCID: PMC11185855
- DOI: 10.1159/000535514
A Multicenter Phase 2 Trial Evaluating the Efficacy and Safety of Preoperative Lenvatinib Therapy for Patients with Advanced Hepatocellular Carcinoma (LENS-HCC Trial)
Abstract
Introduction: The phase III REFLECT trial demonstrated that lenvatinib was superior to sorafenib in terms of progression-free survival (PFS), time to progression, and objective response rate (ORR) for patients with unresectable hepatocellular carcinoma (HCC). This study assessed the efficacy and safety of preoperative lenvatinib therapy for patients with oncologically or technically unresectable HCC.
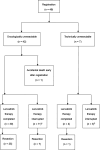
Methods: In this multicenter single-arm phase II trial, patients with advanced HCC and factors suggestive of a poor prognosis (macroscopic vascular invasion, extrahepatic metastasis, or multinodular tumors) were enrolled. Patients with these factors, even with technically resectable HCC, were defined as oncologically unresectable because of the expected poor prognosis after surgery. After 8 weeks of lenvatinib therapy, the patients were assessed for resectability, and tumor resection was performed if the tumor was considered technically resectable. The primary endpoint was the surgical resection rate. The secondary endpoints were the macroscopic curative resection rate, overall survival (OS), ORR, PFS, and the change in the indocyanine green retention rate at 15 min as measured before and after lenvatinib therapy. The trial was registered with the Japan Registry of Clinical Trials (s031190057).
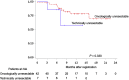
Results: Between July 2019 and January 2021, 49 patients (42 oncologically unresectable patients and 7 technically unresectable patients) from 11 centers were enrolled. The ORR was 37.5% based on mRECIST and 12.5% based on RECIST version 1.1. Thirty-three patients underwent surgery (surgical resection rate: 67.3%) without perioperative mortality. The surgical resection rate was 76.2% for oncologically unresectable patients and 14.3% for technically unresectable patients. The 1-year OS rate and median PFS were 75.9% and 7.2 months, respectively, with a median follow-up period of 9.3 months.
Conclusions: The relatively high surgical resection rate seen in this study suggests the safety and feasibility of lenvatinib therapy followed by surgical resection for patients with oncologically or technically unresectable HCC.
Keywords: Hepatocellular carcinoma; Lenvatinib; Preoperative therapy.
© 2023 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
E.H. reports honoraria from Eisai, Chugai Pharmaceutical, Bayer, Eli Lilly, and Takeda Pharmaceutical. J.S. reports honoraria from Eisai and Chugai Pharmaceutical. K.H. reports honoraria and grants from Eisai, Chugai Pharmaceutical, Bayer, Eli Lilly, and Takeda Pharmaceutical, and research funding from Chugai Pharmaceutical.
Figures


References
-
- Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, et al. . Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol. 2016;65(5):938–43. - PubMed
-
- Kojima H, Hatano E, Taura K, Seo S, Yasuchika K, Uemoto S. Hepatic resection for hepatocellular carcinoma with tumor thrombus in the major portal vein. Dig Surg. 2015;32(6):413–20. - PubMed
-
- Kim DS, Kim BW, Hatano E, Hwang S, Hasegawa K, Kudo A, et al. . Surgical outcomes of hepatocellular carcinoma with bile duct tumor thrombus: a korea-Japan multicenter study. Ann Surg. 2020;271(5):913–21. - PubMed
LinkOut - more resources
Full Text Sources

