Structural Modifications of Covalent Cathepsin S Inhibitors: Impact on Affinity, Selectivity, and Permeability
- PMID: 38894911
- PMCID: PMC11181490
- DOI: 10.1021/acsmedchemlett.4c00050
Structural Modifications of Covalent Cathepsin S Inhibitors: Impact on Affinity, Selectivity, and Permeability
Abstract
Cathepsin S (catS) is a member of the cysteine protease family with limited tissue distribution, which is predominantly found in antigen-presenting cells. Due to overexpression and overactivity of catS in numerous cancers, inhibition of catS is supposed to improve the antitumor response. Here, we explore the potential of small-molecule catS inhibitors emphasizing their in vitro pharmacodynamics and pharmacokinetics. Membrane permeability of selected inhibitors was measured with a Parallel Artificial Membrane Permeation Assay and correlated to calculated physicochemical parameters and inhibition data. The binding kinetics and inhibition types of potent and selective new inhibitors with unexplored warheads were investigated. Our unique approach involves reversible masking of these potent warheads, allowing for further customization without compromising affinity or selectivity. The most promising inhibitors in this study include covalent aldehyde and ketone derivatives reversibly masked as hydrazones as potential candidates for therapeutic interventions targeting catalytic enzymes and modulating the immune response in cancer.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
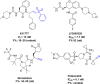
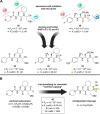
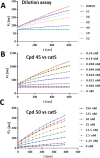


Similar articles
-
Subnanomolar Cathepsin S Inhibitors with High Selectivity: Optimizing Covalent Reversible α-Fluorovinylsulfones and α-Sulfonates as Potential Immunomodulators in Cancer.ChemMedChem. 2023 Aug 1;18(15):e202300160. doi: 10.1002/cmdc.202300160. Epub 2023 Jun 1. ChemMedChem. 2023. PMID: 37222230
-
A road map for prioritizing warheads for cysteine targeting covalent inhibitors.Eur J Med Chem. 2018 Dec 5;160:94-107. doi: 10.1016/j.ejmech.2018.10.010. Epub 2018 Oct 6. Eur J Med Chem. 2018. PMID: 30321804
-
Synthesis of α-fluorocinnamate derivatives as novel cathepsin S inhibitors with in vitro antiproliferative activity against pancreatic cancer cells.Bioorg Med Chem. 2024 Dec 1;115:117987. doi: 10.1016/j.bmc.2024.117987. Epub 2024 Nov 5. Bioorg Med Chem. 2024. PMID: 39509759
-
Novel Opportunities for Cathepsin S Inhibitors in Cancer Immunotherapy by Nanocarrier-Mediated Delivery.Cells. 2020 Sep 2;9(9):2021. doi: 10.3390/cells9092021. Cells. 2020. PMID: 32887380 Free PMC article. Review.
-
Advances in Cathepsin S Inhibition: Challenges and Breakthroughs in Drug Development.Pathophysiology. 2024 Sep 3;31(3):471-487. doi: 10.3390/pathophysiology31030035. Pathophysiology. 2024. PMID: 39311309 Free PMC article. Review.
References
-
- Edman M. C.; Janga S. R.; Meng Z.; Bechtold M.; Chen A. F.; Kim C.; Naman L.; Sarma A.; Teekappanavar N.; Kim A. Y.; Madrigal S.; Singh S.; Ortiz E.; Christianakis S.; Arkfeld D. G.; Mack W. J.; Heur M.; Stohl W.; Hamm-Alvarez S. F. Increased Cathepsin S Activity Associated with Decreased Protease Inhibitory Capacity Contributes to Altered Tear Proteins in Sjögren’s Syndrome Patients. Sci. Rep. 2018, 8 (1), 11044. 10.1038/s41598-018-29411-9. - DOI - PMC - PubMed
-
- Bararia D.; Hildebrand J. A.; Stolz S.; Haebe S.; Alig S.; Trevisani C. P.; Osorio-Barrios F.; Bartoschek M. D.; Mentz M.; Pastore A.; Gaitzsch E.; Heide M.; Jurinovic V.; Rautter K.; Gunawardana J.; Sabdia M. B.; Szczepanowski M.; Richter J.; Klapper W.; Louissaint A.; Ludwig C.; Bultmann S.; Leonhardt H.; Eustermann S.; Hopfner K. P.; Hiddemann W.; von Bergwelt-Baildon M.; Steidl C.; Kridel R.; Tobin J. W. D.; Gandhi M. K.; Weinstock D. M.; Schmidt-Supprian M.; Sárosi M. B.; Rudelius M.; Passerini V.; Mautner J.; Weigert O. Cathepsin S Alterations Induce a Tumor-Promoting Immune Microenvironment in Follicular Lymphoma. Cell Rep. 2020, 31 (5), 107522. 10.1016/j.celrep.2020.107522. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

