Conjugated Nonionic Detergent Micelles: An Efficient Purification Platform for Dimeric Human Immunoglobulin A
- PMID: 38894919
- PMCID: PMC11181477
- DOI: 10.1021/acsmedchemlett.4c00128
Conjugated Nonionic Detergent Micelles: An Efficient Purification Platform for Dimeric Human Immunoglobulin A
Abstract
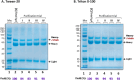
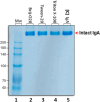
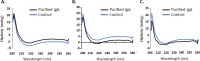
The SARS-COV-2 virus is a deadly agent of inflammatory respiratory disease. Since 2020, studies have focused on developing new therapies based on galactose-rich IgA antibodies. Clinical surveys have also revealed that galactose-deficient IgA1 polymerizes in serum, producing IgA nephropathy, which is a common cause of kidney failure in young adults. Here we show that IgA1-IgA2 dimers are efficiently and economically purified in solution via conjugated nonionic surfactant micellar aggregates. Quantitative capture at pH 7 and extraction at pH 6.5 can avoid antibody exposure to acidic, potentially denaturing conditions. Brij-O20 aggregates lead to the highest process yields (88-91%) and purity (94%). Recovered IgA dimers preserve their native secondary structure and do not self-associate. Increasing the reaction volume has little impact on yield or purity. By introducing an efficient, inexpensive IgA purification protocol, we assist pharmaceutical firms and research laboratories in developing new IgA-based therapies as well as in increasing our understanding of IgA1 polymerization.
© 2024 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Efficient separation of IgG from IgM antibodies via conjugated surfactant micelles.J Chromatogr B Analyt Technol Biomed Life Sci. 2023 Jul 15;1226:123805. doi: 10.1016/j.jchromb.2023.123805. Epub 2023 Jun 17. J Chromatogr B Analyt Technol Biomed Life Sci. 2023. PMID: 37354733
-
A general platform for antibody purification utilizing engineered-micelles.MAbs. 2019 Apr;11(3):583-592. doi: 10.1080/19420862.2019.1565749. Epub 2019 Feb 6. MAbs. 2019. PMID: 30618334 Free PMC article.
-
Detergent micelle conjugates containing amino acid monomers allow purification of human IgG near neutral pH.J Chromatogr B Analyt Technol Biomed Life Sci. 2022 Aug 15;1206:123358. doi: 10.1016/j.jchromb.2022.123358. Epub 2022 Jun 28. J Chromatogr B Analyt Technol Biomed Life Sci. 2022. PMID: 35780745
-
Pathogenesis of IgA Nephropathy: Current Understanding and Implications for Development of Disease-Specific Treatment.J Clin Med. 2021 Sep 29;10(19):4501. doi: 10.3390/jcm10194501. J Clin Med. 2021. PMID: 34640530 Free PMC article. Review.
-
Paradigm shift in activity assessment of IgA nephropathy - optimizing the next generation of diagnostic and therapeutic maneuvers via glycan targeting.Expert Opin Biol Ther. 2015 Apr;15(4):583-93. doi: 10.1517/14712598.2015.1006624. Epub 2015 Jan 20. Expert Opin Biol Ther. 2015. PMID: 25604055 Review.
Cited by
-
Purification of Human Immunoglobulin G with Bathophenanthroline-Zn2+, -Fe2+, or -Cu2+ Complexes.Antibodies (Basel). 2025 May 12;14(2):40. doi: 10.3390/antib14020040. Antibodies (Basel). 2025. PMID: 40407692 Free PMC article.
References
-
- Bioley G.; Monnerat J.; Lötscher M.; Vonarburg C.; Zuercher A.; Corthésy B. Plasma-Derived Polyreactive Secretory-Like IgA and IgM Opsonizing Salmonella enterica Typhimurium Reduces Invasion and Gut Tissue Inflammation through Agglutination. Front. Immunol. 2017, 8, 1043. 10.3389/fimmu.2017.01043. - DOI - PMC - PubMed
-
- Pasquier B.; Launay P.; Kanamaru Y.; Moura I. C.; Pfirsch S.; Ruffié C.; Hénin D.; Benhamou M.; Pretolani M.; Blank U.; Monteiro R. C. Identification of FcalphaRI as an inhibitory receptor that controls inflammation: dual role of FcRgamma ITAM. Immunity 2005, 22 (1), 31–42. 10.1016/j.immuni.2004.11.017. - DOI - PubMed
-
- Rossato E.; Ben Mkaddem S.; Kanamaru Y.; Hurtado-Nedelec M.; Hayem G.; Descatoire V.; Vonarburg C.; Miescher S.; Zuercher A. W.; Monteiro R. C. Reversal of Arthritis by Human Monomeric IgA Through the Receptor-Mediated SH2 Domain-Containing Phosphatase 1 Inhibitory Pathway. Arthritis Rheumatol. 2015, 67 (7), 1766–77. 10.1002/art.39142. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

