Novel 2,4-Dichloro-5-sulfamoylbenzoic Acid Oxime Esters: First Studies as Potential Human Carbonic Anhydrase Inhibitors
- PMID: 38894925
- PMCID: PMC11181484
- DOI: 10.1021/acsmedchemlett.4c00206
Novel 2,4-Dichloro-5-sulfamoylbenzoic Acid Oxime Esters: First Studies as Potential Human Carbonic Anhydrase Inhibitors
Abstract
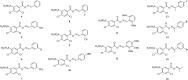
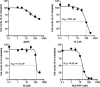
In this study, a focused library of oxime ester derivatives of 2,4-dichloro-5-sulfamoylbenzoic acid (lasamide) containing Schiff bases was synthesized and tested in vitro for their ability to inhibit the cytosolic human carbonic anhydrases (hCAs) I and II, as well as the transmembrane and tumor-associated IX and XII isoforms. As a result, we obtained a first line of knowledge on lasamide derivatives potentially useful for development as CA inhibitors (CAIs). In particular, we focused our attention on the derivative 11, which was selective toward hCAs IX and XII over the cytosolic isoenzymes. An in silico study was conducted to assess the binding mode of 11 within hCAs IX and XII. Also, antiproliferative assays highlighted promising derivatives. The data obtained in this study are currently in use for the development of better-performing compounds on the tumor-associated isoforms.
© 2024 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
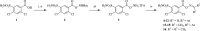

Similar articles
-
Discovery of novel benzenesulfonamides incorporating 1,2,3-triazole scaffold as carbonic anhydrase I, II, IX, and XII inhibitors.Int J Biol Macromol. 2023 Jun 1;239:124232. doi: 10.1016/j.ijbiomac.2023.124232. Epub 2023 Mar 30. Int J Biol Macromol. 2023. PMID: 37001773
-
Inhibition of carbonic anhydrase isoforms I, II, IX and XII with novel Schiff bases: identification of selective inhibitors for the tumor-associated isoforms over the cytosolic ones.Bioorg Med Chem. 2014 Nov 1;22(21):5883-90. doi: 10.1016/j.bmc.2014.09.021. Epub 2014 Sep 21. Bioorg Med Chem. 2014. PMID: 25267005
-
Carbonic anhydrase inhibitors. Inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes I, II, IX, and XII with Schiff's bases incorporating chromone and aromatic sulfonamide moieties, and their zinc complexes.Bioorg Med Chem Lett. 2005 Jun 15;15(12):3096-101. doi: 10.1016/j.bmcl.2005.04.055. Bioorg Med Chem Lett. 2005. PMID: 15908204
-
Structural investigations on coumarins leading to chromeno[4,3-c]pyrazol-4-ones and pyrano[4,3-c]pyrazol-4-ones: New scaffolds for the design of the tumor-associated carbonic anhydrase isoforms IX and XII.Eur J Med Chem. 2018 Feb 25;146:47-59. doi: 10.1016/j.ejmech.2018.01.033. Epub 2018 Jan 12. Eur J Med Chem. 2018. PMID: 29407972
-
Novel 2-(hydrazinocarbonyl)-3-phenyl-1H-indole-5-sulfonamide based thiosemicarbazides as potent and selective inhibitors of tumor-associated human carbonic anhydrase IX and XII: Synthesis, cytotoxicity, and molecular modelling studies.Bioorg Chem. 2024 Mar;144:107096. doi: 10.1016/j.bioorg.2024.107096. Epub 2024 Jan 23. Bioorg Chem. 2024. PMID: 38290186
Cited by
-
Lasamide Containing Sulfonylpiperazines as Effective Agents for the Management of Glaucoma Associated Symptoms.ChemMedChem. 2024 Dec 16;19(24):e202400601. doi: 10.1002/cmdc.202400601. Epub 2024 Nov 8. ChemMedChem. 2024. PMID: 39319579 Free PMC article.
References
-
- Nerella S. G.; Thacker P. S.; Arifuddin M.; Supuran C. T. Tumor Associated Carbonic Anhydrase Inhibitors: Rational Approaches, Design Strategies, Structure Activity Relationship and Mechanistic Insights. European Journal of Medicinal Chemistry Reports 2024, 10, 100131 10.1016/j.ejmcr.2024.100131. - DOI
-
- Angeli A.; Chelli I.; Lucarini L.; Sgambellone S.; Marri S.; Villano S.; Ferraroni M.; De Luca V.; Capasso C.; Carta F.; Supuran C. T. Novel Carbonic Anhydrase Inhibitors with Dual-Tail Core Sulfonamide Show Potent and Lasting Effects for Glaucoma Therapy. J. Med. Chem. 2024, 67 (4), 3066–3089. 10.1021/acs.jmedchem.3c02254. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

