Stepwise Nitric Oxide Release and Antibacterial Activity of a Nitric Oxide Photodonor Hosted within Cyclodextrin Branched Polymer Nanocarriers
- PMID: 38894929
- PMCID: PMC11181500
- DOI: 10.1021/acsmedchemlett.4c00061
Stepwise Nitric Oxide Release and Antibacterial Activity of a Nitric Oxide Photodonor Hosted within Cyclodextrin Branched Polymer Nanocarriers
Abstract
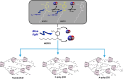
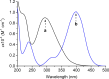
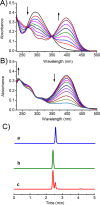
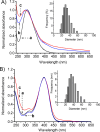
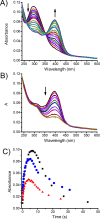
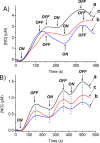
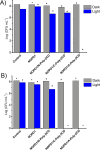
A hydrophobic nitric oxide (NO) photodonor integrating both nitroso and nitro functionalities within its chromophoric skeleton has been synthesized. Excitation of this compound with blue light triggers the release of two NO molecules from the nitroso and the nitro functionalities via a stepwise mechanism. Encapsulation of the NO photodonor within biocompatible neutral, cationic, and anionic β-cyclodextrin branched polymers as suitable carriers leads to supramolecular nanoassemblies, which exhibit the same nature of the photochemical processes but NO photorelease performances enhanced by about 1 order of magnitude when compared with the free guest. Antibacterial tests carried out with methicillin-resistant Staphylococcus aureus and Acinetobacter baumannii demonstrate an effective antibacterial activity exclusively under light activation and point out a differentiated role of the polymeric nanocarriers in determining the outcome of the antibacterial photodynamic action.
© 2024 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Ignarro L. J.Nitric Oxide: Biology and Pathobiology; Elsevier: St. Louis, 2010.
-
- Tewari D.; Sah A. N.; Bawari S.; Nabavi S. F.; Dehpour A. R.; Shirooie S.; Braidy N.; Fiebich B. L.; Vacca R. A.; Nabavi S. M. Role of nitric oxide in neurodegeneration: function, regulation, and inhibition. Curr. Neuropharmacol. 2020, 19, 114–126. 10.2174/1570159X18666200429001549. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

