Propionic acid promotes neurite recovery in damaged multiple sclerosis neurons
- PMID: 38894951
- PMCID: PMC11184351
- DOI: 10.1093/braincomms/fcae182
Propionic acid promotes neurite recovery in damaged multiple sclerosis neurons
Abstract
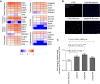
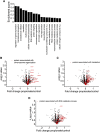
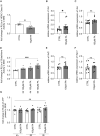
Neurodegeneration in the autoimmune disease multiple sclerosis still poses a major therapeutic challenge. Effective drugs that target the inflammation can only partially reduce accumulation of neurological deficits and conversion to progressive disease forms. Diet and the associated gut microbiome are currently being discussed as crucial environmental risk factors that determine disease onset and subsequent progression. In people with multiple sclerosis, supplementation of the short-chain fatty acid propionic acid, as a microbial metabolite derived from the fermentation of a high-fiber diet, has previously been shown to regulate inflammation accompanied by neuroprotective properties. We set out to determine whether the neuroprotective impact of propionic acid is a direct mode of action of short-chain fatty acids on CNS neurons. We analysed neurite recovery in the presence of the short-chain fatty acid propionic acid and butyric acid in a reverse-translational disease-in-a-dish model of human-induced primary neurons differentiated from people with multiple sclerosis-derived induced pluripotent stem cells. We found that recovery of damaged neurites is induced by propionic acid and butyric acid. We could also show that administration of butyric acid is able to enhance propionic acid-associated neurite recovery. Whole-cell proteome analysis of induced primary neurons following recovery in the presence of propionic acid revealed abundant changes of protein groups that are associated with the chromatin assembly, translational, and metabolic processes. We further present evidence that these alterations in the chromatin assembly were associated with inhibition of histone deacetylase class I/II following both propionic acid and butyric acid treatment, mediated by free fatty acid receptor signalling. While neurite recovery in the presence of propionic acid is promoted by activation of the anti-oxidative response, administration of butyric acid increases neuronal ATP synthesis in people with multiple sclerosis-specific induced primary neurons.
Keywords: gut microbiome metabolites; microbiome–gut–brain axis; neurite recovery; neurodegeneration.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
R.G. and A.H. have filed a patent on the supportive immunomodulatory effect of C3–C8 aliphatic fatty acids.
Figures




References
-
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–1517. - PubMed
-
- Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15(9):545–558. - PubMed
-
- Friese MA, Schattling B, Fugger L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat Rev Neurol. 2014;10(4):225–238. - PubMed
-
- Baecher-Allan C, Kaskow BJ, Weiner HL. Multiple sclerosis: Mechanisms and immunotherapy. Neuron. 2018;97(4):742–768. - PubMed
-
- Faissner S, Plemel JR, Gold R, Yong VW. Progressive multiple sclerosis: From pathophysiology to therapeutic strategies. Nat Rev Drug Discov. 2019;18(12):905–922. - PubMed
LinkOut - more resources
Full Text Sources
