Effectiveness of two-dose vs. one-dose varicella vaccine in children in Shanghai, China: a prospective cohort study
- PMID: 38894987
- PMCID: PMC11183296
- DOI: 10.3389/fpubh.2024.1320407
Effectiveness of two-dose vs. one-dose varicella vaccine in children in Shanghai, China: a prospective cohort study
Abstract
Objective: Varicella, a highly contagious viral disease caused by the varicella-zoster virus (VZV), affects millions globally, with a higher prevalence among children. After the initial infection, VZV lies dormant in sensory ganglia and has the potential to reactivate much later, causing herpes zoster (HZ). Vaccination is one of the most effective methods to prevent varicella, and the two-dose varicella vaccine (VarV) regimen is widely used around the world. In China, the VarV has been included in the national immunization programme with a recommended single-dose regimen. This study aimed to compare the effectiveness of the two-dose vs. one-dose VarV regimen in children in Shanghai, China.
Materials and methods: A prospective cohort study was conducted in Shanghai, China, from September 2018 to December 2022. The study enrolled children aged 3-18 years who had received either the one-dose, two-dose, or 0-dose VarV regimen. Vaccination history, varicella infection status, and relevant variables, including demographic information (name, date of birth and sex) and medical history (clinical features of varicella and illness duration) were collected through medical record review and parental interviews.
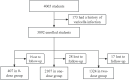
Results: A total of 3,838 children were included in the study, with 407 in the 0-dose regimen group, 2,107 in the one-dose regimen group and 1,324 in the two-dose regimen group. The corresponding incidence density in these groups was 0.13, 0.05 and 0.03 cases per 1,000 person-days, respectively. The adjusted vaccine effectiveness (VE) was 81.7% (95%CI: 59.3-91.8%) for the two-dose regimen and 60.3% (95%CI: 29.3-77.7%) for the one-dose regimen, compared to the 0-dose regimen. The two-dose VarV regimen showed a protective effectiveness of 47.6% (95%CI: 2.5-71.9%) compared to the one-dose VarV regimen.
Conclusion: This study provides evidence supporting the greater effectiveness of the two-dose VarV regimen in preventing varicella infection compared to the one-dose regimen.
Keywords: child; cohort; effectiveness; two-dose; varicella vaccine.
Copyright © 2024 Li, Xu, Liu, Teng, Liang and Wang.
Similar articles
-
Effectiveness of second-dose varicella vaccination as post-exposure prophylaxis: a prospective cohort study.Clin Microbiol Infect. 2019 Jul;25(7):872-877. doi: 10.1016/j.cmi.2018.11.013. Epub 2018 Nov 23. Clin Microbiol Infect. 2019. PMID: 30472425
-
Impact of a two-dose varicella immunization program on the incidence of varicella: a multi-year observational study in Shanghai, China.Expert Rev Vaccines. 2021 Sep;20(9):1177-1183. doi: 10.1080/14760584.2021.1963236. Epub 2021 Aug 11. Expert Rev Vaccines. 2021. PMID: 34343035
-
Seroepidemiology of varicella in Hangzhou, China in the vaccine era.Hum Vaccin Immunother. 2018;14(10):2464-2471. doi: 10.1080/21645515.2018.1477909. Epub 2018 Jul 30. Hum Vaccin Immunother. 2018. PMID: 30019992 Free PMC article.
-
Varicella-zoster virus: pathogenesis, incidence patterns and vaccination programs.Minerva Pediatr. 2016 Jun;68(3):213-25. Minerva Pediatr. 2016. PMID: 27125440 Review.
-
[Systematic reviews and evidence quality assessment on effectiveness of 1 dose varicella attenuated live vaccine for healthy children aged 1-12 years in China].Zhonghua Liu Xing Bing Xue Za Zhi. 2020 Jul 10;41(7):1138-1144. doi: 10.3760/cma.j.cn112338-20191025-00762. Zhonghua Liu Xing Bing Xue Za Zhi. 2020. PMID: 32741184 Chinese.
Cited by
-
Changes in Epidemiological Characteristics of Varicella and Breakthrough Cases in Ningbo, China, From 2010 to 2023: Surveillance Study.JMIR Public Health Surveill. 2025 Jun 18;11:e71691. doi: 10.2196/71691. JMIR Public Health Surveill. 2025. PMID: 40533066 Free PMC article.
-
Analysis of sero-epidemiological characteristics of varicella in healthy children in Wuxi, China.Hum Vaccin Immunother. 2024 Dec 31;20(1):2432118. doi: 10.1080/21645515.2024.2432118. Epub 2024 Dec 5. Hum Vaccin Immunother. 2024. PMID: 39635720 Free PMC article.
References
-
- Varicella and herpes zoster vaccines: WHO position paper, June 2014. Rel Epidemiol Hebdom. (2014) 89:265–87. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical