n-Bu4NI/K2S2O8 Mediated Csp2-Csp2 Bond Cleavage - Transformylation from p-Anisaldehyde to Primary Amides
- PMID: 38895098
- PMCID: PMC11182648
- DOI: 10.1002/adsc.202301505
n-Bu4NI/K2S2O8 Mediated Csp2-Csp2 Bond Cleavage - Transformylation from p-Anisaldehyde to Primary Amides
Abstract
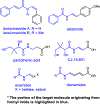
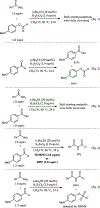
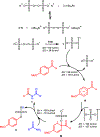
n-Bu4NI/K2S2O8 mediated transformylation from p-anisaldehyde to primary amides is reported. The mechanistic studies suggest the reaction occurs via a single electron transfer pathway. Based on the DFT electronic structure calculations of various reaction pathways, the most plausible mechanism involves the formation of a phenyl radical cation and an arenium ion as the key intermediates. It represents the first example where p-anisaldehyde is employed as a formyl source via a non-metal mediated Csp2-Csp2 bond cleavage.
Keywords: Csp2-Csp2 bond cleavage; N-formyl imide; n-Bu4NI/K2S2O8 mediated; single electron transfer reaction; transformylation.
Figures
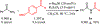
References
Grants and funding
LinkOut - more resources
Full Text Sources
