Fibrotic pathways and fibroblast-like synoviocyte phenotypes in osteoarthritis
- PMID: 38895122
- PMCID: PMC11183113
- DOI: 10.3389/fimmu.2024.1385006
Fibrotic pathways and fibroblast-like synoviocyte phenotypes in osteoarthritis
Abstract
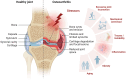
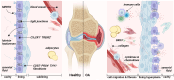
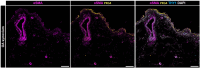
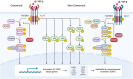
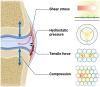
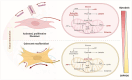
Osteoarthritis (OA) is the most common form of arthritis, characterized by osteophyte formation, cartilage degradation, and structural and cellular alterations of the synovial membrane. Activated fibroblast-like synoviocytes (FLS) of the synovial membrane have been identified as key drivers, secreting humoral mediators that maintain inflammatory processes, proteases that cause cartilage and bone destruction, and factors that drive fibrotic processes. In normal tissue repair, fibrotic processes are terminated after the damage has been repaired. In fibrosis, tissue remodeling and wound healing are exaggerated and prolonged. Various stressors, including aging, joint instability, and inflammation, lead to structural damage of the joint and micro lesions within the synovial tissue. One result is the reduced production of synovial fluid (lubricants), which reduces the lubricity of the cartilage areas, leading to cartilage damage. In the synovial tissue, a wound-healing cascade is initiated by activating macrophages, Th2 cells, and FLS. The latter can be divided into two major populations. The destructive thymocyte differentiation antigen (THY)1─ phenotype is restricted to the synovial lining layer. In contrast, the THY1+ phenotype of the sublining layer is classified as an invasive one with immune effector function driving synovitis. The exact mechanisms involved in the transition of fibroblasts into a myofibroblast-like phenotype that drives fibrosis remain unclear. The review provides an overview of the phenotypes and spatial distribution of FLS in the synovial membrane of OA, describes the mechanisms of fibroblast into myofibroblast activation, and the metabolic alterations of myofibroblast-like cells.
Keywords: TGF-β; fibroblast to myofibroblast transition; fibrosis; inflammation; mechanical stress; metabolism; osteoarthritis; senescence.
Copyright © 2024 Damerau, Rosenow, Alkhoury, Buttgereit and Gaber.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
-
- Senthelal S, Li J, Goyal A, Bansal P, Thomas MA. Arthritis. Treasure Island (FL: StatPearls; (2021).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

