Urinary complement biomarkers in immune-mediated kidney diseases
- PMID: 38895123
- PMCID: PMC11184941
- DOI: 10.3389/fimmu.2024.1357869
Urinary complement biomarkers in immune-mediated kidney diseases
Abstract
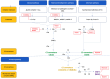
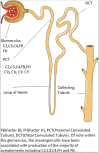
The complement system, an important part of the innate system, is known to play a central role in many immune mediated kidney diseases. All parts of the complement system including the classical, alternative, and mannose-binding lectin pathways have been implicated in complement-mediated kidney injury. Although complement components are thought to be mainly synthesized in the liver and activated in the circulation, emerging data suggest that complement is synthesized and activated inside the kidney leading to direct injury. Urinary complement biomarkers are likely a better reflection of inflammation within the kidneys as compared to traditional serum complement biomarkers which may be influenced by systemic inflammation. In addition, urinary complement biomarkers have the advantage of being non-invasive and easily accessible. With the rise of therapies targeting the complement pathways, there is a critical need to better understand the role of complement in kidney diseases and to develop reliable and non-invasive biomarkers to assess disease activity, predict treatment response and guide therapeutic interventions. In this review, we summarized the current knowledge on urinary complement biomarkers of kidney diseases due to immune complex deposition (lupus nephritis, primary membranous nephropathy, IgA nephropathy) and due to activation of the alternative pathway (C3 glomerulopathy, thrombotic microangiography, ANCA-associated vasculitis). We also address the limitations of current research and propose future directions for the discovery of urinary complement biomarkers.
Keywords: C3 glomerulopathy; IgA nephropathy; complement; complement drug; kidney disease; lupus nephritis (LN); primary membranous nephropathy; urine biomarker.
Copyright © 2024 Kesarwani, Bukhari, Kahlenberg and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous