IL-1 and TNF mediates IL-6 signaling at the maternal-fetal interface during intrauterine inflammation
- PMID: 38895127
- PMCID: PMC11183269
- DOI: 10.3389/fimmu.2024.1416162
IL-1 and TNF mediates IL-6 signaling at the maternal-fetal interface during intrauterine inflammation
Abstract
Introduction: IL6 signaling plays an important role in triggering labor and IL6 is an established biomarker of intrauterine infection/inflammation (IUI) driven preterm labor (PTL). The biology of IL6 during IUI at the maternal-fetal interface was investigated in samples from human subjects and non-human primates (NHP).
Methods: Pregnant women with histologic chorioamnionitis diagnosed by placenta histology were recruited (n=28 term, n=43 for preterm pregnancies from 26-36 completed weeks of gestation). IUI was induced in Rhesus macaque by intraamniotic injection of lipopolysachharide (LPS, n=23). IL1 signaling was blocked using Anakinra (human IL-1 receptor antagonist, n=13), and Tumor necrosis factor (TNF) signaling was blocked by anti TNF-antibody (Adalimumab n=14). The blockers were given before LPS. All animals including controls (intraamniotic injection of saline n=27), were delivered 16h after LPS/saline exposure at about 80% gestation.
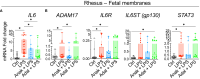
Results: IUI induced a robust expression of IL6 mRNAs in the fetal membranes (chorion-amnion-decidua tissue) both in humans (term and preterm) and NHP. The major sources of IL6 mRNA expression were the amnion mesenchymal cells (AMC) and decidua stroma cells. Additionally, during IUI in the NHP, ADAM17 (a protease that cleaves membrane bound IL6 receptor (IL6R) to release a soluble form) and IL6R mRNA increased in the fetal membranes, and the ratio of IL6 and soluble forms of IL6R, gp130 increased in the amniotic fluid signifying upregulation of IL6 trans-signaling. Both IL1 and TNF blockade suppressed LPS-induced IL6 mRNAs in the AMC and variably decreased elements of IL6 trans-signaling.
Discussion: These data suggest that IL1 and TNF blockers may be useful anti-inflammatory agents via suppression of IL6 signaling at the maternal-fetal interface.
Keywords: amnion; chorioamnionitis; inflammation; innate immunity; reproductive immunology.
Copyright © 2024 Presicce, Roland, Senthamaraikannan, Cappelletti, Hammons, Miller, Jobe, Chougnet, DeFranco and Kallapur.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Venkatesh KK, Leviton A, Hecht JL, Joseph RM, Douglass LM, Frazier JA, et al. . Histologic chorioamnionitis and risk of neurodevelopmental impairment at age 10 years among extremely preterm infants born before 28 weeks of gestation. Am J Obstet Gynecol. (2020) 223(5):745.e1–745.e10. doi: 10.1016/j.ajog.2020.05.001 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

