Periductal Mastitis, a Disease with Distinct Clinicopathological Features from Granulomatous Lobular Mastitis
- PMID: 38895142
- PMCID: PMC11185250
- DOI: 10.2147/JIR.S464585
Periductal Mastitis, a Disease with Distinct Clinicopathological Features from Granulomatous Lobular Mastitis
Abstract
Purpose: Periductal mastitis (PDM) is a chronic inflammatory lesion of the breast with an unknown etiology, and it is difficult for clinicians to differentiate it from granulomatous lobular mastitis (GLM), although they have different treatment strategies and prognosis. This study aimed to investigate the differences in their clinicopathologic features to inform treatment strategies.
Patients and methods: Between 2011 and 2020, 121 patients diagnosed with PDM and 57 patients with GLM were retrospective analysis. Patient data were extracted on demographics, clinical presentation, pathologic characteristics, treatments and clinical response. Histopathological evaluations were performed on core needle biopsy specimens. Immunohistochemical stains using antibodies against CD3, CD4, CD8, CD20, and CD138 was performed to define immune cell infiltration.
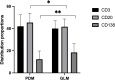
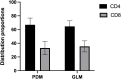
Results: PDM patients had a higher median age compared to GLM patients (38 vs 32, p<0.001). PDM was primarily located in the areolar area, while GLM predominantly affected the peripheral quadrant of the breast (56.20% vs 75.44%, p<0.001). Histopathologically, more ductal dilatation (90.08% vs 3.51%, p<0.001), ductal wall thickening (47.93% vs 1.75%, p<0.001), and ductal rupture (44.63% vs 5.26%, p<0.001) were observed in PDM. GLM presented with significantly more granuloma (94.74% vs 10.74%, p<0.001), microabscess (68.42% vs 28.93%, p<0.001), and lipid vacuole (40.35% vs 8.26%, p<0.001) formation than PDM. Immunohistochemical analysis revealed a significant presence of CD20+ B lymphocytes in PDM and a higher prevalence of CD8+ T lymphocytes in GLM, indicating differing immune responses. Treatment outcomes varied, with PDM patients responding well to surgery and anti-mycobacterial therapy, while GLM patients showed favorable responses to steroid therapy.
Conclusion: PDM is a specific entity with a similar clinical presentation but distinct histopathological features and immune profiles to GLM. Further research is needed to elucidate the pathogenesis and optimize therapeutic approaches for these breast inflammatory conditions.
Keywords: etiology; granulomatous lobular mastitis; immunology; pathology; periductal mastitis.
© 2024 Zhou et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


Similar articles
-
Development of a machine learning-based diagnostic model using hematological parameters to differentiate periductal mastitis from granulomatous lobular mastitis.Sci Prog. 2025 Apr-Jun;108(2):368504251333513. doi: 10.1177/00368504251333513. Epub 2025 Apr 13. Sci Prog. 2025. PMID: 40223288 Free PMC article.
-
Identification of periductal mastitis and granulomatous lobular mastitis: a literature review.Ann Transl Med. 2023 Feb 15;11(3):158. doi: 10.21037/atm-22-6473. Epub 2023 Feb 3. Ann Transl Med. 2023. PMID: 36846004 Free PMC article. Review.
-
Clinicopathological features of granulomatous lobular mastitis and mammary duct ectasia.Oncol Lett. 2020 Jan;19(1):840-848. doi: 10.3892/ol.2019.11156. Epub 2019 Nov 28. Oncol Lett. 2020. PMID: 31885718 Free PMC article.
-
Pathological manifestations of granulomatous lobular mastitis.Front Med (Lausanne). 2024 Feb 2;11:1326587. doi: 10.3389/fmed.2024.1326587. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38371511 Free PMC article. Review.
-
[Granulomatous lobular mastitis: a clinicopathological analysis of 300 cases].Zhonghua Bing Li Xue Za Zhi. 2019 Mar 8;48(3):231-236. doi: 10.3760/cma.j.issn.0529-5807.2019.03.012. Zhonghua Bing Li Xue Za Zhi. 2019. PMID: 30831651 Chinese.
Cited by
-
Single-Cell RNA Sequencing Reveals the Immune Landscape of Granulomatous Mastitis.Inflammation. 2025 May 8. doi: 10.1007/s10753-025-02310-8. Online ahead of print. Inflammation. 2025. PMID: 40338490
References
LinkOut - more resources
Full Text Sources
Research Materials

