Sensitize Tumor Immunotherapy: Immunogenic Cell Death Inducing Nanosystems
- PMID: 38895146
- PMCID: PMC11184231
- DOI: 10.2147/IJN.S457782
Sensitize Tumor Immunotherapy: Immunogenic Cell Death Inducing Nanosystems
Abstract
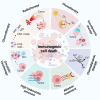
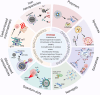
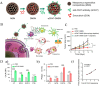
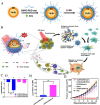
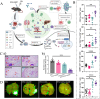
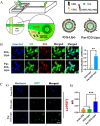
Low immunogenicity of tumors poses a challenge in the development of effective tumor immunotherapy. However, emerging evidence suggests that certain therapeutic approaches, such as chemotherapy, radiotherapy, and phototherapy, can induce varying degrees of immunogenic cell death (ICD). This ICD phenomenon leads to the release of tumor antigens and the maturation of dendritic cells (DCs), thereby enhancing tumor immunogenicity and promoting immune responses. However, the use of a single conventional ICD inducer often fails to achieve in situ tumor ablation and establish long-term anti-tumor immune responses. Furthermore, the induction of ICD induction varies among different approaches, and the distribution of the therapeutic agent within the body influences the level of ICD and the occurrence of toxic side effects. To address these challenges and further boost tumor immunity, researchers have explored nanosystems as inducers of ICD in combination with tumor immunotherapy. This review examines the mechanisms of ICD and different induction methods, with a specific focus on the relationship between ICD and tumor immunity. The aim is to explore the research advancements utilizing various nanomaterials to enhance the body's anti-tumor effects by inducing ICD. This paper aims to contribute to the development and clinical application of nanomaterial-based ICD inducers in the field of cancer immunotherapy by providing important theoretical guidance and practical references.
Keywords: DAMPs; ICD; immunogenicity; nanosystems; tumor immunotherapy.
© 2024 Peng et al.
Conflict of interest statement
The authors declare no conflicts of interests in this work.
Figures




Similar articles
-
Nanoparticle-mediated immunogenic cell death for cancer immunotherapy.Int J Pharm. 2024 May 10;656:124045. doi: 10.1016/j.ijpharm.2024.124045. Epub 2024 Mar 30. Int J Pharm. 2024. PMID: 38561134 Review.
-
Immunogenic cell death-based cancer vaccines: promising prospect in cancer therapy.Front Immunol. 2024 Apr 29;15:1389173. doi: 10.3389/fimmu.2024.1389173. eCollection 2024. Front Immunol. 2024. PMID: 38745666 Free PMC article. Review.
-
Inorganic Nanobiomaterials Boost Tumor Immunotherapy: Strategies and Applications.Acc Chem Res. 2025 Apr 15;58(8):1210-1223. doi: 10.1021/acs.accounts.4c00843. Epub 2025 Apr 3. Acc Chem Res. 2025. PMID: 40179239 Review.
-
Synergizing autophagic cell death and oxaliplatin-induced immunogenic death by a self-delivery micelle for enhanced tumor immunotherapy.Acta Biomater. 2024 Dec;190:548-559. doi: 10.1016/j.actbio.2024.10.025. Epub 2024 Oct 18. Acta Biomater. 2024. PMID: 39426655
-
Recent progress in stimuli-responsive nanosystems for inducing immunogenic cell death.J Control Release. 2021 Sep 10;337:505-520. doi: 10.1016/j.jconrel.2021.07.038. Epub 2021 Jul 24. J Control Release. 2021. PMID: 34314800 Review.
Cited by
-
Harnessing the power of traceable system C-GAP: homologous-targeting to fire up T-cell immune responses with low-dose irradiation.J Nanobiotechnology. 2025 Mar 12;23(1):207. doi: 10.1186/s12951-025-03281-6. J Nanobiotechnology. 2025. PMID: 40075499 Free PMC article.
-
Galactose-modified erythrocyte membrane fusion liposomes enable the targeted delivery of drug nanoparticles to the liver.RSC Adv. 2025 May 29;15(22):17781-17794. doi: 10.1039/d4ra07489k. eCollection 2025 May 21. RSC Adv. 2025. PMID: 40443690 Free PMC article.
-
Preclinical advance in nanoliposome-mediated photothermal therapy in liver cancer.Lipids Health Dis. 2025 Jan 31;24(1):31. doi: 10.1186/s12944-024-02429-x. Lipids Health Dis. 2025. PMID: 39891269 Free PMC article. Review.
-
Identification of a novel immunogenic cell death-related classifier to predict prognosis and optimize precision treatment in hepatocellular carcinoma.Heliyon. 2025 Jan 7;11(2):e41380. doi: 10.1016/j.heliyon.2024.e41380. eCollection 2025 Jan 30. Heliyon. 2025. PMID: 39897773 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

