Pharmacokinetics and Bioequivalence of Apremilast Tablets in Chinese Healthy Subjects Under Fasting and Postprandial States
- PMID: 38895175
- PMCID: PMC11185170
- DOI: 10.2147/DDDT.S461771
Pharmacokinetics and Bioequivalence of Apremilast Tablets in Chinese Healthy Subjects Under Fasting and Postprandial States
Abstract
Objective: This study compared the pharmacokinetics, safety and bioequivalence (BE) of generic and original apremilast tablets in healthy Chinese subjects under fasting and postprandial conditions, providing sufficient evidence for abbreviated new drug application.
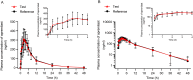
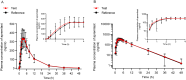
Methods: A randomized, open-label, two-formulation, single-dose, two-period crossover pharmacokinetic study was performed. Thirty-two eligible healthy Chinese subjects were enrolled in fasting and postprandial studies, respectively. In each trial, subjects received a single 30-mg dose of the test or reference apremilast tablet, followed by a 7-day washout interval between periods. Serial blood samples were obtained for up to 48 h post-intake in each period, and the plasma concentrations of apremilast were determined by a validated method. The primary pharmacokinetic (PK) parameters, including the maximum plasma concentration (Cmax), the areas under the plasma concentration-time curve (AUC0-t, AUC0-∞), were calculated using the non-compartmental method. The geometric mean ratios of the two formulations and the corresponding 90% confidence intervals (CIs) were acquired for bioequivalence analysis. The safety of both formulations was also evaluated.
Results: Under fasting and postprandial states, the PK parameters of the test drug were similar to those of the reference drug. The 90% CIs of the geometric mean ratios of the test to reference formulations were 94.09-103.44% for Cmax, 94.05-103.51% for AUC0-t, and 94.56-103.86% for AUC0-∞ under fasting conditions, and 99.18-112.48% for Cmax, 98.79-106.02% for AUC0-t, and 98.95-105.89% for AUC0-∞ under postprandial conditions, all of which were within the bioequivalence range of 80.00-125.00%. Both formulations were well tolerated, and no serious adverse events occurred during the study.
Conclusion: The trial confirmed that the PK parameters of the generic and original apremilast tablets were bioequivalent in healthy Chinese subjects under fasting and postprandial states, which met the predetermined regulatory standards. Both formulations were safe and well tolerated.
Clinical trial registration: chinaDrugtrials.org.cn, identifier CTR20191056 (July 30, 2019); chictr.org.cn, identifier ChiCTR2300076806 (October 19, 2023).
Keywords: apremilast; bioequivalence; pharmacokinetics; psoriasis; safety.
© 2024 Bai et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest in this study.
Figures
Similar articles
-
Pharmacokinetics and Bioequivalence of Two Formulations of Azithromycin Tablets: A Randomized, Single-Dose, Three-Period, Crossover Study in Healthy Chinese Volunteers Under Fasting and Fed Conditions.Drugs R D. 2024 Jun;24(2):201-209. doi: 10.1007/s40268-024-00464-8. Epub 2024 May 30. Drugs R D. 2024. PMID: 38811485 Free PMC article. Clinical Trial.
-
Evaluating the pharmacokinetics and safety of blonanserin tablets and Lonasen®: a randomized, open-label, two-period, two-sequence, self-crossover phase I clinical trial.Front Pharmacol. 2025 Jan 3;15:1511214. doi: 10.3389/fphar.2024.1511214. eCollection 2024. Front Pharmacol. 2025. PMID: 39976233 Free PMC article.
-
Bioequivalence Study of Vortioxetine Hydrobromide Tablets in Healthy Chinese Subjects Under Fasting and Fed Conditions.Drug Des Devel Ther. 2023 Sep 29;17:3035-3046. doi: 10.2147/DDDT.S428771. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 37795495 Free PMC article. Clinical Trial.
-
Simultaneous confidence regions for multivariate bioequivalence.Stat Med. 2017 Dec 20;36(29):4585-4603. doi: 10.1002/sim.7446. Epub 2017 Aug 30. Stat Med. 2017. PMID: 28857229 Review.
-
Partial AUCs in Long-Acting Injectables: Rationale, Challenges, Variability, Usefulness, and Clinical Relevance.Pharmaceutics. 2024 Dec 26;17(1):21. doi: 10.3390/pharmaceutics17010021. Pharmaceutics. 2024. PMID: 39861670 Free PMC article. Review.
Cited by
-
The treatment of psoriasis via herbal formulation and nano-polyherbal formulation: A new approach.Bioimpacts. 2024 Aug 11;15:30341. doi: 10.34172/bi.30341. eCollection 2025. Bioimpacts. 2024. PMID: 40256226 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

