This is a preprint.
Diminished Immune Cell Adhesion in Hypoimmune ICAM-1 Knockout Pluripotent Stem Cells
- PMID: 38895244
- PMCID: PMC11185752
- DOI: 10.1101/2024.06.07.597791
Diminished Immune Cell Adhesion in Hypoimmune ICAM-1 Knockout Pluripotent Stem Cells
Update in
-
Diminished immune cell adhesion in hypoimmune ICAM-1 knockout human pluripotent stem cells.Nat Commun. 2025 Aug 12;16(1):7415. doi: 10.1038/s41467-025-62568-2. Nat Commun. 2025. PMID: 40796566 Free PMC article.
Abstract
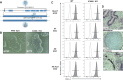
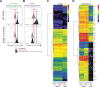
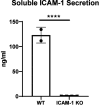
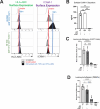
Hypoimmune gene edited human pluripotent stem cells (hPSCs) are a promising platform for developing reparative cellular therapies that evade immune rejection. Existing first-generation hypoimmune strategies have used CRISPR/Cas9 editing to modulate genes associated with adaptive (e.g., T cell) immune responses, but have largely not addressed the innate immune cells (e.g., monocytes, neutrophils) that mediate inflammation and rejection processes occurring early after graft transplantation. We identified the adhesion molecule ICAM-1 as a novel hypoimmune target that plays multiple critical roles in both adaptive and innate immune responses post-transplantation. In a series of studies, we found that ICAM-1 blocking or knock-out (KO) in hPSC-derived cardiovascular therapies imparted significantly diminished binding of multiple immune cell types. ICAM-1 KO resulted in diminished T cell proliferation responses in vitro and in longer in vivo retention/protection of KO grafts following immune cell encounter in NeoThy humanized mice. The ICAM-1 KO edit was also introduced into existing first-generation hypoimmune hPSCs and prevented immune cell binding, thereby enhancing the overall hypoimmune capacity of the cells. This novel hypoimmune editing strategy has the potential to improve the long-term efficacy and safety profiles of regenerative therapies for cardiovascular pathologies and a number of other diseases.
Conflict of interest statement
Conflict of Interest Disclosure M.E.B is a consultant for Taconic Biosciences, and is an inventor on a patent application filed by WARF related to this work. The other authors declare no conflict of interest.
Figures




Similar articles
-
Diminished immune cell adhesion in hypoimmune ICAM-1 knockout human pluripotent stem cells.Nat Commun. 2025 Aug 12;16(1):7415. doi: 10.1038/s41467-025-62568-2. Nat Commun. 2025. PMID: 40796566 Free PMC article.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Derivation of trophoblast stem cells from naïve human pluripotent stem cells.Elife. 2020 Feb 12;9:e52504. doi: 10.7554/eLife.52504. Elife. 2020. PMID: 32048992 Free PMC article.
-
Stem cell transplantation for induction of remission in medically refractory Crohn's disease.Cochrane Database Syst Rev. 2022 May 13;5(5):CD013070. doi: 10.1002/14651858.CD013070.pub2. Cochrane Database Syst Rev. 2022. PMID: 35556242 Free PMC article.
References
-
- Yu J. et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science 318, 1917–1920 (2007). - PubMed
-
- Takahashi K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007). - PubMed
-
- Yan W. et al. Stem cell-based therapy in cardiac repair after myocardial infarction: Promise, challenges, and future directions. J. Mol. Cell. Cardiol. 188, 1–14 (2024). - PubMed
-
- Ramzy A. et al. Implanted pluripotent stem-cell-derived pancreatic endoderm cells secrete glucose-responsive C-peptide in patients with type 1 diabetes. Cell Stem Cell 28, 2047–2061.e5 (2021). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
