This is a preprint.
A frameshift mutation in the murine Prkra gene causes dystonia and exhibits abnormal cerebellar development and reduced eIF2α phosphorylation
- PMID: 38895245
- PMCID: PMC11185611
- DOI: 10.1101/2024.06.04.597421
A frameshift mutation in the murine Prkra gene causes dystonia and exhibits abnormal cerebellar development and reduced eIF2α phosphorylation
Update in
-
Mutation in Prkra results in cerebellar abnormality and reduced eIF2α phosphorylation in a model of DYT-PRKRA.Dis Model Mech. 2024 Nov 1;17(11):dmm050929. doi: 10.1242/dmm.050929. Epub 2024 Nov 26. Dis Model Mech. 2024. PMID: 39512178 Free PMC article.
Abstract
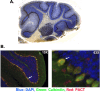
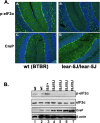
Mutations in Prkra gene, which encodes PACT/RAX cause early onset primary dystonia DYT-PRKRA, a movement disorder that disrupts coordinated muscle movements. PACT/RAX activates protein kinase R (PKR, aka EIF2AK2) by a direct interaction in response to cellular stressors to mediate phosphorylation of the α subunit of the eukaryotic translation initiation factor 2 (eIF2α). Mice homozygous for a naturally arisen, recessively inherited frameshift mutation, Prkra lear-5J exhibit progressive dystonia. In the present study, we investigate the biochemical and developmental consequences of the Prkra lear-5J mutation. Our results indicate that the truncated PACT/RAX protein retains its ability to interact with PKR, however, it inhibits PKR activation. Furthermore, mice homozygous for the mutation have abnormalities in the cerebellar development as well as a severe lack of dendritic arborization of Purkinje neurons. Additionally, reduced eIF2α phosphorylation is noted in the cerebellums and Purkinje neurons of the homozygous Prkra lear-5J mice. These results indicate that PACT/RAX mediated regulation of PKR activity and eIF2α phosphorylation plays a role in cerebellar development and contributes to the dystonia phenotype resulting from this mutation.
Keywords: DYT-PRKRA; EIF2AK2; PACT/RAX; PKR; cerebellum; dystonia.
Conflict of interest statement
Financial Disclosure/Conflict of Interest: The authors declare no financial disclosures or conflicts of interest.
Figures


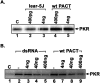



References
-
- Barber G. N. (2001) Host defense, viruses and apoptosis, Cell Death Differ 8(2): 113–26. - PubMed
-
- Beauvais G., Rodriguez-Losada N., Ying L., Zakirova Z., Watson J. L., Readhead B., Gadue P., French D. L., Ehrlich M. E. and Gonzalez-Alegre P. (2018) Exploring the Interaction Between eIF2alpha Dysregulation, Acute Endoplasmic Reticulum Stress and DYT1 Dystonia in the Mammalian Brain, Neuroscience 371: 455–468. - PubMed
-
- Bellato H. M. and Hajj G. N. (2016) Translational control by eIF2α in neurons: Beyond the stress response, Cytoskeleton (Hoboken) 73(10): 551–565. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
