This is a preprint.
Mechanism of an Intrinsic Oscillation in Rat Geniculate Interneurons
- PMID: 38895250
- PMCID: PMC11185623
- DOI: 10.1101/2024.06.06.597830
Mechanism of an Intrinsic Oscillation in Rat Geniculate Interneurons
Abstract
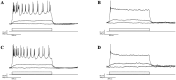
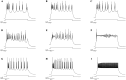
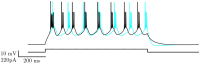
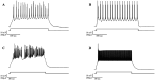
Depolarizing current injections produced a rhythmic bursting of action potentials - a bursting oscillation - in a set of local interneurons in the lateral geniculate nucleus (LGN) of rats. The current dynamics underlying this firing pattern have not been determined, though this cell type constitutes an important cellular component of thalamocortical circuitry, and contributes to both pathologic and non-pathologic brain states. We thus investigated the source of the bursting oscillation using pharmacological manipulations in LGN slices in vitro and in silico. 1. Selective blockade of calcium channel subtypes revealed that high-threshold calcium currents and contributed strongly to the oscillation. 2. Increased extracellular K+ concentration (decreased K+currents) eliminated the oscillation. 3. Selective blockade of K+ channel subtypes demonstrated that the calcium-sensitive potassium current ( ) was of primary importance. A morphologically simplified, multicompartment model of the thalamic interneuron characterized the oscillation as follows: 1. The low-threshold calcium current provided the strong initial burst characteristic of the oscillation. 2. Alternating fluxes through high-threshold calcium channels and then provided the continuing oscillation's burst and interburst periods respectively. This interplay between and contrasts with the current dynamics underlying oscillations in thalamocortical and reticularis neurons, which primarily involve and , or and respectively. These findings thus point to a novel electrophysiological mechanism for generating intrinsic oscillations in a major thalamic cell type. Because local interneurons can sculpt the behavior of thalamocortical circuits, these results suggest new targets for the manipulation of ascending thalamocortical network activity.
Figures




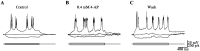


Similar articles
-
An intrinsic oscillation in interneurons of the rat lateral geniculate nucleus.J Neurophysiol. 1999 Feb;81(2):702-11. doi: 10.1152/jn.1999.81.2.702. J Neurophysiol. 1999. PMID: 10036271
-
Burst firing in identified rat geniculate interneurons.Neuroscience. 1999;91(4):1445-60. doi: 10.1016/s0306-4522(98)00665-4. Neuroscience. 1999. PMID: 10391450
-
Ionic mechanisms underlying synchronized oscillations and propagating waves in a model of ferret thalamic slices.J Neurophysiol. 1996 Sep;76(3):2049-70. doi: 10.1152/jn.1996.76.3.2049. J Neurophysiol. 1996. PMID: 8890314
-
On the action of the anti-absence drug ethosuximide in the rat and cat thalamus.J Neurosci. 1998 Jul 1;18(13):4842-53. doi: 10.1523/JNEUROSCI.18-13-04842.1998. J Neurosci. 1998. PMID: 9634550 Free PMC article.
-
Novel vistas of calcium-mediated signalling in the thalamus.Pflugers Arch. 2004 May;448(2):131-8. doi: 10.1007/s00424-003-1234-5. Epub 2004 Feb 10. Pflugers Arch. 2004. PMID: 14770314 Review.
References
-
- Acuna-Goycolea C., Brenowitz S. D., and Regehr W. G. (2008). Active dendritic conductances dynamically regulate GABA release from thalamic interneurons. Neuron, 57(3):420–431. - PubMed
-
- Adams D. J. and Berecki G. (2013). Mechanisms of conotoxin inhibition of n-type (cav2.2) calcium channels. Biochimica et Biophysica Acta (BBA) - Biomembranes, 1828(7):1619–1628. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
