This is a preprint.
The distribution of beneficial mutational effects between two sister yeast species poorly explains natural outcomes of vineyard adaptation
- PMID: 38895255
- PMCID: PMC11185594
- DOI: 10.1101/2024.06.03.597243
The distribution of beneficial mutational effects between two sister yeast species poorly explains natural outcomes of vineyard adaptation
Update in
-
The distribution of beneficial mutational effects between two sister yeast species poorly explains natural outcomes of vineyard adaptation.Genetics. 2024 Oct 7;228(4):iyae160. doi: 10.1093/genetics/iyae160. Online ahead of print. Genetics. 2024. PMID: 39373582 Free PMC article.
Abstract
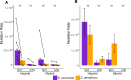
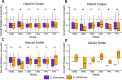
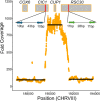
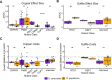
Domesticated strains of Saccharomyces cerevisiae have adapted to resist copper and sulfite, two chemical stressors commonly used in winemaking. S. paradoxus, has not adapted to these chemicals despite being consistently present in sympatry with S. cerevisiae in vineyards. This contrast represents a case of apparent evolutionary constraints favoring greater adaptive capacity in S. cerevisiae. In this study, we used a comparative mutagenesis approach to test whether S. paradoxus is mutationally constrained with respect to acquiring greater copper and sulfite resistance. For both species, we assayed the rate, effect size, and pleiotropic costs of resistance mutations and sequenced a subset of 150 mutants isolated from our screen. We found that the distributions of mutational effects displayed by the two species were very similar and poorly explained the natural pattern. We also found that chromosome VIII aneuploidy and loss of function mutations in PMA1 confer copper resistance in both species, whereas loss of function mutations in REG1 were only a viable route to copper resistance in S. cerevisiae. We also observed a single de novo duplication of the CUP1 gene in S. paradoxus but none in S. cerevisiae. For sulfite, loss of function mutations in RTS1 and KSP1 confer resistance in both species, but mutations in RTS1 have larger average effects in S. paradoxus. Our results show that even when the distributions of mutational effects are largely similar, species can differ in the adaptive paths available to them. They also demonstrate that assays of the distribution of mutational effects may lack predictive insight concerning adaptive outcomes.
Keywords: Saccharomyces cerevisiae; Saccharomyces paradoxus; copper; distribution of mutational effects; phenotype assays; sulfite; whole genome sequencing.
Figures



References
-
- Adamo GM, Lotti M, Tamas MJ, Brocca S. 2012. Amplification of the CUP1 gene is associated with evolution of copper tolerance in Saccharomyces cerevisiae. Microbiology. 158(9):2325–35. - PubMed
-
- Alexander RM. 1985. The ideal and the feasible: physical constraints on evolution. Biol J Linn Soc. 26(4):345–58.
-
- Almeida P, Barbosa R, Bensasson D, Gonçalves P, Sampaio JP. 2017. Adaptive divergence in wine yeasts and their wild relatives suggests a prominent role for introgressions and rapid evolution at noncoding sites. Mol Ecol. 26(7):2167–82. - PubMed
-
- Almeida P, Barbosa R, Zalar P, Imanishi Y, Shimizu K, Turchetti B, Legras J, Serra M, Dequin S, Couloux A, et al. 2015. A population genomics insight into the Mediterranean origins of wine yeast domestication. Mol Ecol. 24(21):5412–27. - PubMed
-
- Amerine MA, Berg HW, Cruness WV. 1972. The technology of wine making 3rd edition. AVI Publishing Company, West Port. Connecticut.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
