This is a preprint.
Arterial effects of anthracycline: structural and inflammatory assessments in non-human primates and lymphoma patients using 18F-FDG positron emission tomography
- PMID: 38895275
- PMCID: PMC11185566
- DOI: 10.1101/2024.05.30.596741
Arterial effects of anthracycline: structural and inflammatory assessments in non-human primates and lymphoma patients using 18F-FDG positron emission tomography
Update in
-
Arterial effects of anthracycline: structural and inflammatory assessments in non-human primates and lymphoma patients.Clin Sci (Lond). 2025 Jan 15;139(1):29-41. doi: 10.1042/CS20241529. Clin Sci (Lond). 2025. PMID: 39680089 Free PMC article.
Abstract
Background: Anthracyclines, such as doxorubicin, are important anti-cancer therapies but are associated with arterial injury. Histopathological insights have been limited to small animal models and the role of inflammation in the arterial toxic effects of anthracycline is unclear in humans. Our aims were: 1) To evaluate aortic media fibrosis and injury in non-human primates treated with anthracyclines; 2) To assess the effect of anthracycline on aortic inflammation in patients treated for lymphoma.
Methods: 1) African Green monkeys (AGM) received doxorubicin (30-60 mg/m2/biweekly IV, cumulative dose: 240 mg/m2). Blinded histopathologic analyses of collagen deposition and cell vacuolization in the ascending aorta were performed 15 weeks after the last doxorubicin dose and compared to 5 age- and gender-matched healthy, untreated AGMs. 2) Analysis of the thoracic aorta of patients with diffuse large B-cell lymphoma (DLBCL), at baseline and after doxorubicin exposure, was performed using 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) in this observational study. The primary outcome was change in maximal tissue-to-background ratio (TBRmax) of the thoracic aorta from baseline to their end-of-treatment clinical PET/CT.
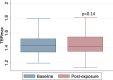
Results: In AGMs, doxorubicin exposure was associated with greater aortic fibrosis (collagen deposition: doxorubicin cohort 6.23±0.88% vs. controls 4.67±0.54%; p=0.01) and increased intracellular vacuolization (doxorubicin 66.3 ± 10.1 vs controls 11.5 ± 4.2 vacuoles/field, p<0.0001) than untreated controls.In 101 patients with DLBCL, there was no change in aortic TBRmax after anthracycline exposure (pre-doxorubicin TBRmax 1.46±0.16 vs post-doxorubicin TBRmax 1.44±0.14, p=0.14). The absence of change in TBRmax was consistent across all univariate analyses.
Conclusions: In a large animal model, anthracycline exposure was associated with aortic fibrosis. In patients with lymphoma, anthracycline exposure was not associated with aortic inflammation.Further research is required to elucidate the mechanisms of anthracycline-related vascular harm.
Keywords: PET; anthracycline; doxorubicin; fibrosis; lymphoma; vascular toxicity.
Figures


Similar articles
-
Arterial effects of anthracycline: structural and inflammatory assessments in non-human primates and lymphoma patients.Clin Sci (Lond). 2025 Jan 15;139(1):29-41. doi: 10.1042/CS20241529. Clin Sci (Lond). 2025. PMID: 39680089 Free PMC article.
-
FDG PET/CT for evaluating systemic arterial inflammation induced by anthracycline-based chemotherapy of Hodgkin lymphoma: A retrospective cohort study.Medicine (Baltimore). 2020 Nov 25;99(48):e23259. doi: 10.1097/MD.0000000000023259. Medicine (Baltimore). 2020. PMID: 33235083 Free PMC article.
-
Appearance of aseptic vascular grafts after endovascular aortic repair on [(18)F]fluorodeoxyglucose positron emission tomography/computed tomography.World J Radiol. 2023 Aug 28;15(8):241-249. doi: 10.4329/wjr.v15.i8.241. World J Radiol. 2023. PMID: 37662425 Free PMC article.
-
Prognostic Importance of Bone Marrow Uptake on Baseline 18F-FDG Positron Emission Tomography in Diffuse Large B Cell Lymphoma.Cancer Biother Radiopharm. 2016 Dec;31(10):361-365. doi: 10.1089/cbr.2016.2132. Cancer Biother Radiopharm. 2016. PMID: 27996313
-
The Canadian Study of Arterial Inflammation in Patients with Diabetes and Recent Vascular Events, Evaluation of Colchicine Effectiveness (CADENCE): protocol for a randomised, double-blind, placebo-controlled trial.BMJ Open. 2023 Nov 10;13(11):e074463. doi: 10.1136/bmjopen-2023-074463. BMJ Open. 2023. PMID: 37949621 Free PMC article.
References
-
- Tan TC, Neilan TG, Francis S, Plana JC, Scherrer-Crosbie M. Anthracycline-Induced Cardiomyopathy in Adults. In: Comprehensive Physiology. Wiley; 2015. p. 1517–1540. - PubMed
-
- Narayan HK, French B, Khan AM, Plappert T, Hyman D, Bajulaiye A, Domchek S, DeMichele A, Clark A, Matro J, et al. Noninvasive Measures of Ventricular-Arterial Coupling and Circumferential Strain Predict Cancer Therapeutics–Related Cardiac Dysfunction. JACC Cardiovasc. Imaging. 2016;9:1131–1141. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
