This is a preprint.
Loss of mitochondrial pyruvate transport initiates cardiac glycogen accumulation and heart failure
- PMID: 38895296
- PMCID: PMC11185624
- DOI: 10.1101/2024.06.06.597841
Loss of mitochondrial pyruvate transport initiates cardiac glycogen accumulation and heart failure
Abstract
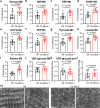
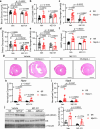
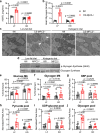
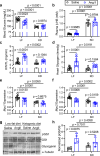
Heart failure involves metabolic alterations including increased glycolysis despite unchanged or decreased glucose oxidation. The mitochondrial pyruvate carrier (MPC) regulates pyruvate entry into the mitochondrial matrix, and cardiac deletion of the MPC in mice causes heart failure. How MPC deletion results in heart failure is unknown. We performed targeted metabolomics and isotope tracing in wildtype (fl/fl) and cardiac-specific Mpc2-/- (CS-Mpc2-/-) hearts after in vivo injection of U-13C-glucose. Failing CS-Mpc2-/- hearts contained normal levels of ATP and phosphocreatine, yet these hearts displayed increased enrichment from U-13C-glucose and increased glycolytic metabolite pool sizes. 13C enrichment and pool size was also increased for the glycogen intermediate UDP-glucose, as well as increased enrichment of the glycogen pool. Glycogen levels were increased ~6-fold in the failing CS-Mpc2-/- hearts, and glycogen granules were easily detected by electron microscopy. In young, non-failing CS-Mpc2-/- hearts, increased glycolytic 13C enrichment occurred, but glycogen levels remained low and unchanged compared to fl/fl hearts. Inhibiting glycogen synthase with MZ-101 reduced cardiac glycogen levels and improved heart failure. Feeding a ketogenic diet to CS-Mpc2-/- mice reversed the heart failure and normalized the cardiac glycogen and glycolytic metabolite accumulation. Cardiac glycogen levels were also elevated in mice infused with angiotensin-II, and both the cardiac hypertrophy and glycogen levels were improved by ketogenic diet. Thus, loss of MPC in the heart causes glycogen accumulation and heart failure, while inhibition of glycogen synthesis or a ketogenic diet can reverse both the glycogen accumulation and heart failure.
Keywords: heart failure; ketogenic diet; mitochondria; pyruvate.
Conflict of interest statement
Disclosures G.J.P. has a research collaboration agreement with Thermo Fisher Scientific and is a scientific advisor for Cambridge Isotope Laboratories. K.S.M. receives research support from Cirius Therapeutics and serves as a consultant for BioGenerator Ventures. All other authors declare no conflicts of interest.
Figures



Similar articles
-
Ketone Body Metabolism is Not Required for Improvement of Heart Failure by Ketogenic Diet in Mice.bioRxiv [Preprint]. 2024 Sep 2:2024.08.30.610511. doi: 10.1101/2024.08.30.610511. bioRxiv. 2024. PMID: 39282341 Free PMC article. Preprint.
-
MULTIPLE, REDUNDANT CARBOXYLIC ACID TRANSPORTERS SUPPORT MITOCHONDRIAL METABOLISM IN PLASMODIUM FALCIPARUM.bioRxiv [Preprint]. 2024 Nov 27:2024.11.26.624872. doi: 10.1101/2024.11.26.624872. bioRxiv. 2024. Update in: J Biol Chem. 2025 Jul;301(7):110248. doi: 10.1016/j.jbc.2025.110248. PMID: 39651245 Free PMC article. Updated. Preprint.
-
Structure of human mitochondrial pyruvate carrier MPC1 and MPC2 complex.Nat Commun. 2025 Jul 21;16(1):6700. doi: 10.1038/s41467-025-61939-z. Nat Commun. 2025. PMID: 40691140 Free PMC article.
-
Maternal and neonatal outcomes of elective induction of labor.Evid Rep Technol Assess (Full Rep). 2009 Mar;(176):1-257. Evid Rep Technol Assess (Full Rep). 2009. PMID: 19408970 Free PMC article.
-
WITHDRAWN: Interventions for fatigue and weight loss in adults with advanced progressive illness.Cochrane Database Syst Rev. 2017 Apr 7;4(4):CD008427. doi: 10.1002/14651858.CD008427.pub3. Cochrane Database Syst Rev. 2017. PMID: 28387447 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
