This is a preprint.
Adeno-associated viral delivery of Env-specific antibodies prevents SIV rebound after discontinuing antiretroviral therapy
- PMID: 38895320
- PMCID: PMC11185534
- DOI: 10.1101/2024.05.30.593694
Adeno-associated viral delivery of Env-specific antibodies prevents SIV rebound after discontinuing antiretroviral therapy
Update in
-
Adeno-associated viral delivery of Env-specific antibodies prevents SIV rebound after discontinuing antiretroviral therapy.Sci Immunol. 2025 Feb 28;10(104):eadq4973. doi: 10.1126/sciimmunol.adq4973. Epub 2025 Feb 28. Sci Immunol. 2025. PMID: 40020046 Free PMC article.
Abstract
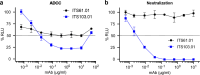
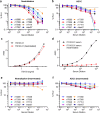
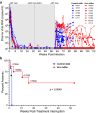
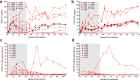
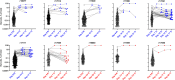
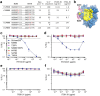
An alternative to lifelong antiretroviral therapy (ART) is needed to achieve durable control of HIV-1. Here we show that adeno-associated virus (AAV)-delivery of two rhesus macaque antibodies to the SIV envelope glycoprotein (Env) with potent neutralization and antibody-dependent cellular cytotoxicity can prevent viral rebound in macaques infected with barcoded SIVmac239M after discontinuing suppressive ART. Following AAV administration, sustained antibody expression with minimal anti-drug antibody responses was achieved in all but one animal. After ART withdrawal, SIV replication rebounded within two weeks in all of the control animals but remained below the threshold of detection in plasma (<15 copies/mL) for more than a year in four of the eight animals that received AAV vectors encoding Env-specific antibodies. Viral sequences from animals with delayed rebound exhibited restricted barcode diversity and antibody escape. Thus, sustained expression of antibodies with potent antiviral activity can afford durable, ART-free containment of pathogenic SIV infection.
Conflict of interest statement
Competing interests Authors declare that they have no competing interests.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
