This is a preprint.
Multi-Modality Deep Infarct: Non-invasive identification of infarcted myocardium using composite in-silico-human data learning
- PMID: 38895325
- PMCID: PMC11185550
- DOI: 10.1101/2024.05.31.596513
Multi-Modality Deep Infarct: Non-invasive identification of infarcted myocardium using composite in-silico-human data learning
Abstract
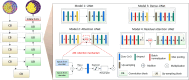
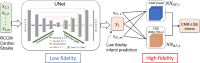
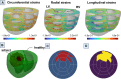
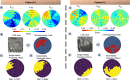
Myocardial infarction (MI) continues to be a leading cause of death worldwide. The precise quantification of infarcted tissue is crucial to diagnosis, therapeutic management, and post-MI care. Late gadolinium enhancement-cardiac magnetic resonance (LGE-CMR) is regarded as the gold standard for precise infarct tissue localization in MI patients. A fundamental limitation of LGE-CMR is the invasive intravenous introduction of gadolinium-based contrast agents that present potential high-risk toxicity, particularly for individuals with underlying chronic kidney diseases. Herein, we develop a completely non-invasive methodology that identifies the location and extent of an infarct region in the left ventricle via a machine learning (ML) model using only cardiac strains as inputs. In this transformative approach, we demonstrate the remarkable performance of a multi-fidelity ML model that combines rodent-based in-silico-generated training data (low-fidelity) with very limited patient-specific human data (high-fidelity) in predicting LGE ground truth. Our results offer a new paradigm for developing feasible prognostic tools by augmenting synthetic simulation-based data with very small amounts of in-vivo human data. More broadly, the proposed approach can significantly assist with addressing biomedical challenges in healthcare where human data are limited.
Figures




Similar articles
-
Multi-Modality Deep Infarct: Non-invasive identification of infarcted myocardium using composite in-silico-human data learning.Res Sq [Preprint]. 2024 Jun 5:rs.3.rs-4468678. doi: 10.21203/rs.3.rs-4468678/v1. Res Sq. 2024. PMID: 38883756 Free PMC article. Preprint.
-
Non-Invasive Diagnosis of Chronic Myocardial Infarction via Composite In-Silico-Human Data Learning.Adv Sci (Weinh). 2025 Jun 19:e06933. doi: 10.1002/advs.202406933. Online ahead of print. Adv Sci (Weinh). 2025. PMID: 40536227
-
Feasibility of detecting myocardial infarction in the sheep fetus using late gadolinium enhancement CMR imaging.J Cardiovasc Magn Reson. 2017 Sep 13;19(1):69. doi: 10.1186/s12968-017-0383-1. J Cardiovasc Magn Reson. 2017. PMID: 28903760 Free PMC article.
-
Machine Learning-Based Segmentation of Left Ventricular Myocardial Fibrosis from Magnetic Resonance Imaging.Curr Cardiol Rep. 2020 Jun 19;22(8):65. doi: 10.1007/s11886-020-01321-1. Curr Cardiol Rep. 2020. PMID: 32562100 Review.
-
[Cardiac magnetic resonance imaging and the myocardium : Differentiation between vital and nonvital tissue].Herzschrittmacherther Elektrophysiol. 2022 Sep;33(3):272-277. doi: 10.1007/s00399-022-00874-8. Epub 2022 Jul 4. Herzschrittmacherther Elektrophysiol. 2022. PMID: 35781833 Review. German.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
