This is a preprint.
Intracellular pH dynamics respond to extracellular matrix stiffening and mediate vasculogenic mimicry through β-catenin
- PMID: 38895391
- PMCID: PMC11185592
- DOI: 10.1101/2024.06.04.597454
Intracellular pH dynamics respond to extracellular matrix stiffening and mediate vasculogenic mimicry through β-catenin
Update in
-
Intracellular pH dynamics respond to extracellular matrix stiffening and mediate vasculogenic mimicry through β-catenin.Cell Death Dis. 2025 Oct 13;16(1):720. doi: 10.1038/s41419-025-08014-z. Cell Death Dis. 2025. PMID: 41083461 Free PMC article.
Abstract
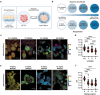
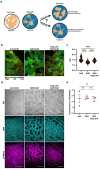
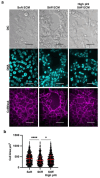
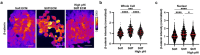
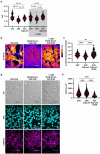
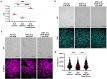
Dysregulated intracellular pH (pHi) dynamics and an altered tumor microenvironment have emerged as drivers of cancer cell phenotypes. However, the molecular integration between the physical properties of the microenvironment and dynamic intracellular signaling responses remains unclear. Here, we identify a mechanistic link between ECM stiffness and pHi dynamics in driving vasculogenic mimicry (VM), an aggressive cancer phenotype associated with poor prognosis. We performed single-cell imaging of pHi in lung and breast metastatic cell lines cultured on tunable-stiffness hydrogel systems. We used two tunable-stiffness hydrogel systems to independently model stiffness induced by increased protein secretion (Matrigel) and increased protein crosslinking (Hyaluronic acid gels). We show that increased ECM stiffness lowers single-cell pHi in both lung and breast metastatic cell lines. We also observed that stiff ECM promotes a distinct morphological phenotype called vasculogenic mimicry (VM). Importantly, we show that low pHi is a necessary mediator of VM, as raising pHi on stiff ECM reduces VM phenotypes. We also find that lowering pHi on soft ECM was sufficient to induce VM in the absence of extracellular stiffening. We characterized β-catenin as a pH-dependent molecular mediator of VM, where stiffness-driven increases in β-catenin abundance can be overridden by high pHi, which destabilizes β-catenin to reduce VM on stiff ECM. In contrast, the transcription factor FOXC2 is activated by ECM stiffness but is insensitive to pHi, and its activity alone is insufficient to maintain VM at high pHi when β-catenin is lost. We uncover a novel mechanotransduction axis in which ECM stiffness regulates intracellular pH to drive β-catenin-induced VM. We also show pHi dynamics can override mechanosensitive cell responses to the extracellular microenvironment. Thus, our work positions pHi as an integrator of mechanotransduction in cancer, suggesting a new framework for therapeutically targeting pHi in cancer and perhaps in other diseases driven by ECM remodeling.
Conflict of interest statement
Competing Interests: Authors declare no competing interests.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
