This is a preprint.
Neurogliaform Cells Exhibit Laminar-specific Responses in the Visual Cortex and Modulate Behavioral State-dependent Cortical Activity
- PMID: 38895403
- PMCID: PMC11185653
- DOI: 10.1101/2024.06.05.597539
Neurogliaform Cells Exhibit Laminar-specific Responses in the Visual Cortex and Modulate Behavioral State-dependent Cortical Activity
Abstract
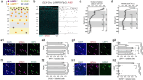
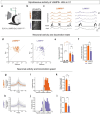
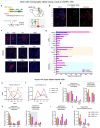
Neurogliaform cells are a distinct type of GABAergic cortical interneurons known for their "volume transmission" output property. However, their activity and function within cortical circuits remain unclear. Here, we developed two genetic tools to target these neurons and examine their function in the primary visual cortex. We found that the spontaneous activity of neurogliaform cells positively correlated with locomotion. Silencing these neurons increased spontaneous activity during locomotion and impaired visual responses in L2/3 pyramidal neurons. Furthermore, the contrast-dependent visual response of neurogliaform cells varies with their laminar location and is constrained by their morphology and input connectivity. These findings demonstrate the importance of neurogliaform cells in regulating cortical behavioral state-dependent spontaneous activity and indicate that their functional engagement during visual stimuli is influenced by their laminar positioning and connectivity.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
