This is a preprint.
Three-dimensional chromatin mapping of sensory neurons reveals that cohesin-dependent genomic domains are required for axonal regeneration
- PMID: 38895406
- PMCID: PMC11185766
- DOI: 10.1101/2024.06.09.597974
Three-dimensional chromatin mapping of sensory neurons reveals that cohesin-dependent genomic domains are required for axonal regeneration
Update in
-
Three-dimensional chromatin mapping of sensory neurons reveals that promoter-enhancer looping is required for axonal regeneration.Proc Natl Acad Sci U S A. 2024 Sep 17;121(38):e2402518121. doi: 10.1073/pnas.2402518121. Epub 2024 Sep 10. Proc Natl Acad Sci U S A. 2024. PMID: 39254997 Free PMC article.
Abstract
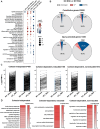
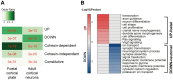
The in vivo three-dimensional genomic architecture of adult mature neurons at homeostasis and after medically relevant perturbations such as axonal injury remains elusive. Here we address this knowledge gap by mapping the three-dimensional chromatin architecture and gene expression programme at homeostasis and after sciatic nerve injury in wild-type and cohesin-deficient mouse sensory dorsal root ganglia neurons via combinatorial Hi-C and RNA-seq. We find that cohesin is required for the full induction of the regenerative transcriptional program, by organising 3D genomic domains required for the activation of regenerative genes. Importantly, loss of cohesin results in disruption of chromatin architecture at regenerative genes and severely impaired nerve regeneration. Together, these data provide an original three-dimensional chromatin map of adult sensory neurons in vivo and demonstrate a role for cohesin-dependent chromatin interactions in neuronal regeneration.
Keywords: 3D chromatin architecture; Axon Regeneration; Epigenetics; Hi-C; cohesion.
Conflict of interest statement
COMPETING INTEREST All the authors declare no competing interests
Figures




References
-
- Beagrie R. A., Pombo A., Gene activation by metazoan enhancers: Diverse mechanisms stimulate distinct steps of transcription. BioEssays : news and reviews in molecular, cellular and developmental biology 38, 881–893 (2016). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
