This is a preprint.
Evaluation in mice of cell-free produced CT584 as a Chlamydia vaccine antigen
- PMID: 38895407
- PMCID: PMC11185655
- DOI: 10.1101/2024.06.04.597210
Evaluation in mice of cell-free produced CT584 as a Chlamydia vaccine antigen
Abstract
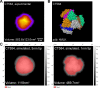
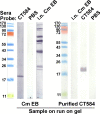
Chlamydia trachomatis is the most prevalent bacterial sexually transmitted pathogen worldwide. Since chlamydial infection is largely asymptomatic with the potential for serious complications, a preventative vaccine is likely the most viable long-term answer to this public health threat. Cell-free protein synthesis (CFPS) utilizes the cellular protein manufacturing machinery decoupled from the requirement for maintaining cellular viability, offering the potential for flexible, rapid, and de-centralized production of recombinant protein vaccine antigens. Here, we use CFPS to produce the putative chlamydial type three secretion system (T3SS) needle-tip protein, CT584, for use as a vaccine antigen in mouse models. High-speed atomic force microscopy (HS-AFM) imaging and computer simulations confirm that CFPS-produced CT584 retains a native-like structure prior to immunization. Female mice were primed with CT584 adjuvanted with CpG-1826 intranasally (i.n.) or CpG-1826 + Montanide ISA 720 intramuscularly (i.m.), followed four-weeks later by an i.m. boost before respiratory challenge with 104 inclusion forming units (IFU) of Chlamydia muridarum. Immunization with CT584 generated robust antibody responses but weak cell mediated immunity and failed to protect against i.n. challenge as demonstrated by body weight loss, increased lungs' weights and the presence of high numbers of IFUs in the lungs. While CT584 alone may not be the ideal vaccine candidate, the speed and flexibility with which CFPS can be used to produce other potential chlamydial antigens makes it an attractive technique for antigen production.
Keywords: CT584; Chlamydia; cell-free protein synthesis; immunization; mice; type-three secretion system; vaccine.
Conflict of interest statement
Conflicts of Interest The authors declare no conflict of interest.
Figures



Similar articles
-
CT584 Is Not a Protective Vaccine Antigen against Respiratory Chlamydial Challenge in Mice.Vaccines (Basel). 2024 Oct 3;12(10):1134. doi: 10.3390/vaccines12101134. Vaccines (Basel). 2024. PMID: 39460301 Free PMC article.
-
A vaccine formulated with a combination of TLR-2 and TLR-9 adjuvants and the recombinant major outer membrane protein elicits a robust immune response and significant protection against a Chlamydia muridarum challenge.Microbes Infect. 2014 Mar;16(3):244-52. doi: 10.1016/j.micinf.2013.11.009. Epub 2013 Nov 27. Microbes Infect. 2014. PMID: 24291713 Free PMC article.
-
Evaluation of Four Adjuvant Combinations, IVAX-1, IVAX-2, CpG-1826+Montanide ISA 720 VG and CpG-1018+Montanide ISA 720 VG, for Safety and for Their Ability to Elicit Protective Immune Responses in Mice against a Respiratory Challenge with Chlamydia muridarum.Pathogens. 2023 Jun 22;12(7):863. doi: 10.3390/pathogens12070863. Pathogens. 2023. PMID: 37513710 Free PMC article.
-
Co-delivery of amphipol-conjugated adjuvant with antigen, and adjuvant combinations, enhance immune protection elicited by a membrane protein-based vaccine against a mucosal challenge with Chlamydia.Vaccine. 2018 Oct 29;36(45):6640-6649. doi: 10.1016/j.vaccine.2018.09.055. Epub 2018 Oct 4. Vaccine. 2018. PMID: 30293763
-
Navigating to the most promising directions amid complex fields of vaccine development: a chlamydial case study.Expert Rev Vaccines. 2019 Dec;18(12):1323-1337. doi: 10.1080/14760584.2019.1698954. Epub 2019 Dec 10. Expert Rev Vaccines. 2019. PMID: 31773996
