This is a preprint.
Inhibition of the Eukaryotic Initiation Factor-2-α Kinase PERK Decreases Risk of Autoimmune Diabetes in Mice
- PMID: 38895427
- PMCID: PMC11185543
- DOI: 10.1101/2023.10.06.561126
Inhibition of the Eukaryotic Initiation Factor-2-α Kinase PERK Decreases Risk of Autoimmune Diabetes in Mice
Update in
-
Inhibition of the eukaryotic initiation factor-2α kinase PERK decreases risk of autoimmune diabetes in mice.J Clin Invest. 2024 Jun 18;134(16):e176136. doi: 10.1172/JCI176136. J Clin Invest. 2024. PMID: 38889047 Free PMC article.
Abstract
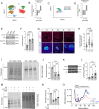
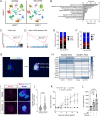
Preventing the onset of autoimmune type 1 diabetes (T1D) is feasible through pharmacological interventions that target molecular stress-responsive mechanisms. Cellular stresses, such as nutrient deficiency, viral infection, or unfolded proteins, trigger the integrated stress response (ISR), which curtails protein synthesis by phosphorylating eIF2α. In T1D, maladaptive unfolded protein response (UPR) in insulin-producing β cells renders these cells susceptible to autoimmunity. We show that inhibition of the eIF2α kinase PERK, a common component of the UPR and ISR, reverses the mRNA translation block in stressed human islets and delays the onset of diabetes, reduces islet inflammation, and preserves β cell mass in T1D-susceptible mice. Single-cell RNA sequencing of islets from PERK-inhibited mice shows reductions in the UPR and PERK signaling pathways and alterations in antigen processing and presentation pathways in β cells. Spatial proteomics of islets from these mice shows an increase in the immune checkpoint protein PD-L1 in β cells. Golgi membrane protein 1, whose levels increase following PERK inhibition in human islets and EndoC-βH1 human β cells, interacts with and stabilizes PD-L1. Collectively, our studies show that PERK activity enhances β cell immunogenicity, and inhibition of PERK may offer a strategy to prevent or delay the development of T1D.
Keywords: Unfolded protein response; integrated stress response; islet; mRNA translation; type 1 diabetes.
Conflict of interest statement
DECLARATION OF INTERESTS VC, MES, DS, and MJM are employees of HiberCell, Inc. SAT, RGM, and HiberCell, Inc. have filed a provisional patent on compounds to inhibit PERK in type 1 diabetes. SAT and RGM received an investigator-initiated award from HiberCell, Inc. for use of PERK inhibitors in this study. KAS is a consultant for and has received research support from HiberCell, Inc. SAO is a co-founder, equity holder, and consultant for OptiKIRA, LLC.
Figures



References
-
- Sims EK, et al. High proinsulin:C-peptide ratio identifies individuals with stage 2 type 1 diabetes at high risk for progression to clinical diagnosis and responses to teplizumab treatment. Diabetologia. [published online ahead of print: September 4, 2023]. 10.1007/s00125-023-06003-5. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
