This is a preprint.
A predominately pulmonary activation of complement in a mouse model of severe COVID-19
- PMID: 38895461
- PMCID: PMC11185570
- DOI: 10.1101/2024.05.31.596892
A predominately pulmonary activation of complement in a mouse model of severe COVID-19
Abstract
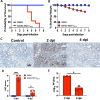
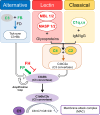
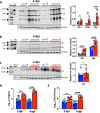
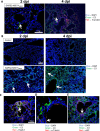
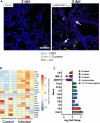
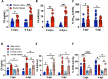
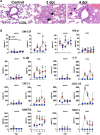
Evidence from in vitro studies and observational human disease data suggest the complement system plays a significant role in SARS-CoV-2 pathogenesis, although how complement dysregulation develops in patients with severe COVID-19 is unknown. Here, using a mouse-adapted SARS-CoV-2 virus (SARS2-N501YMA30) and a mouse model of severe COVID-19, we identify significant serologic and pulmonary complement activation following infection. We observed C3 activation in airway and alveolar epithelia, and in pulmonary vascular endothelia. Our evidence suggests that while the alternative pathway is the primary route of complement activation, components of both the alternative and classical pathways are produced locally by respiratory epithelial cells following infection, and increased in primary cultures of human airway epithelia in response to cytokine exposure. This locally generated complement response appears to precede and subsequently drive lung injury and inflammation. Results from this mouse model recapitulate findings in humans, which suggest sex-specific variance in complement activation, with predilection for increased C3 activity in males, a finding that may correlate with more severe disease. Our findings indicate that complement activation is a defining feature of severe COVID-19 in mice and lay the foundation for further investigation into the role of complement in COVID-19.
Keywords: COVID-19; SARS-CoV-2; complement; inflammation; pulmonary.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
