Influence of β-lactam pharmacodynamics on the systems microbiology of gram-positive and gram-negative polymicrobial communities
- PMID: 38895629
- PMCID: PMC11183306
- DOI: 10.3389/fphar.2024.1339858
Influence of β-lactam pharmacodynamics on the systems microbiology of gram-positive and gram-negative polymicrobial communities
Abstract
Objectives: We sought to evaluate the pharmacodynamics of β-lactam antibacterials against polymicrobial communities of clinically relevant gram-positive and gram-negative pathogens.
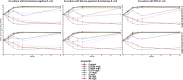
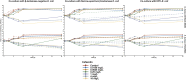
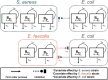
Methods: Two Enterococcus faecalis isolates, two Staphylococcus aureus isolates, and three Escherichia coli isolates with varying β-lactamase production were evaluated in static time-killing experiments. Each gram-positive isolate was exposed to a concentration array of ampicillin (E. faecalis) or cefazolin (S. aureus) alone and during co-culture with an E. coli isolate that was β-lactamase-deficient, produced TEM-1, or produced KPC-3/TEM-1B. The results of the time-killing experiments were summarized using an integrated pharmacokinetic/pharmacodynamics analysis as well as mathematical modelling to fully characterize the antibacterial pharmacodynamics.
Results: In the integrated analysis, the maximum killing of ampicillin (Emax) against both E. faecalis isolates was ≥ 4.11 during monoculture experiments or co-culture with β-lactamase-deficient E. coli, whereas the Emax was reduced to ≤ 1.54 during co-culture with β-lactamase-producing E. coli. In comparison to monoculture experiments, culturing S. aureus with KPC-producing E. coli resulted in reductions of the cefazolin Emax from 3.25 and 3.71 down to 2.02 and 2.98, respectively. Two mathematical models were created to describe the interactions between E. coli and either E. faecalis or S. aureus. When in co-culture with E. coli, S. aureus experienced a reduction in its cefazolin Kmax by 24.8% (23.1%RSE). Similarly, β-lactamase-producing E. coli preferentially protected the ampicillin-resistant E. faecalis subpopulation, reducing Kmax,r by 90.1% (14%RSE).
Discussion: β-lactamase-producing E. coli were capable of protecting S. aureus and E. faecalis from exposure to β-lactam antibacterials.
Keywords: Enterococcus faecalis; Escherichia coli; Staphylococcus aureus; beta-lactam; mechanism-based modeling; pharmacodynamic; polymicrobial; systems microbiology.
Copyright © 2024 Smith, Kaur, Kaur, Minoza, Kent, Barekat and Lenhard.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Capability of Enterococcus faecalis to shield Gram-negative pathogens from aminoglycoside exposure.J Antimicrob Chemother. 2021 Sep 15;76(10):2610-2614. doi: 10.1093/jac/dkab211. J Antimicrob Chemother. 2021. PMID: 34245262 Free PMC article.
-
Interaction of Staphylococcus aureus and Acinetobacter baumannii during In Vitro β-Lactam Exposure.Antimicrob Agents Chemother. 2021 Mar 18;65(4):e02414-20. doi: 10.1128/AAC.02414-20. Print 2021 Mar 18. Antimicrob Agents Chemother. 2021. PMID: 33495215 Free PMC article.
-
Community-Acquired Uropathogenic Escherichia coli, Antimicrobial Susceptibility, and Extended-Spectrum Beta-Lactamase Detection.MEDICC Rev. 2022 May 16;24(2):20-25. doi: 10.37757/mr2022.v24.n2.2. MEDICC Rev. 2022. PMID: 35648059
-
Sulopenem: An Intravenous and Oral Penem for the Treatment of Urinary Tract Infections Due to Multidrug-Resistant Bacteria.Drugs. 2022 Apr;82(5):533-557. doi: 10.1007/s40265-022-01688-1. Epub 2022 Mar 16. Drugs. 2022. PMID: 35294769 Review.
-
Multidrug-Resistant and Virulent Organisms Trauma Infections: Trauma Infectious Disease Outcomes Study Initiative.Mil Med. 2022 May 4;187(Suppl 2):42-51. doi: 10.1093/milmed/usab131. Mil Med. 2022. PMID: 35512375 Free PMC article. Review.
References
-
- Centers for Disease Control (2023). Biggest threats and data. Available at: https://www.cdc.gov/drugresistance/biggest-threats.html (Accessed on July 26, 2023).
-
- WHO (2017). WHO Publishes list of bacteria for which new antibiotics are urgently needed. WHO. Media Centre. News Release. Available at: https://www.who.int/en/news-room/detail/27-02-2017-who-publishes-list-of... (Accessed July 25, 2023).
-
- Athamanolap P., Hsieh K., Wang A. T. (2018). Integrated bacterial identification and antimicrobial susceptibility testing for polymicrobial infections using digital PCR and digital high-resolution melt in a microfluidic array platform. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2018, 5346–5349. 10.1109/EMBC.2018.8513472 - DOI - PubMed
-
- Briaud P., Camus L., Bastien S., Doléans-Jordheim A., Vandenesch F., Moreau K. (2019). Coexistence with Pseudomonas aeruginosa alters Staphylococcus aureus transcriptome, antibiotic resistance and internalization into epithelial cells. Sci. Rep. 9, 16564. 10.1038/s41598-019-52975-z - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

