Real-world treatment patterns of adjuvant endocrine therapy and ovarian suppression in premenopausal HR+/HER2+ breast cancer
- PMID: 38895891
- PMCID: PMC11185945
- DOI: 10.1002/cam4.7317
Real-world treatment patterns of adjuvant endocrine therapy and ovarian suppression in premenopausal HR+/HER2+ breast cancer
Abstract
Background: The optimal adjuvant endocrine therapy (ET) in hormone receptor positive (HR+) and human epidermal growth factor receptor 2 positive (HER2+) premenopausal breast cancer (BC) remains unclear. Moreover, the benefit and clinical indications of ovarian suppression (OS) is poorly elucidated. We described real-world patterns surrounding choice of ET and clinicopathologic features which predicted treatment with OS in a contemporary cohort of premenopausal women with HR+/HER2+ BC.
Methods: This retrospective analysis included premenopausal patients with nonmetastatic HR+/HER2+ BC from the CancerLinQ Discovery database from January 2010 to May 2020. Women were less than 50 years and received chemotherapy, anti-HER2 therapy, and ET. They were categorized into 1 of 4 groups based on type of ET prescribed at initiation: aromatase inhibitor (AI) + OS, OS, tamoxifen + OS, or tamoxifen. Multivariable logistic regression assessed associations between clinicopathologic features and OS use.
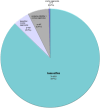
Results: Out of 360,540 patients with BC, 937 were included. The majority (n = 818, 87%) were prescribed tamoxifen, whereas 4 (0.4%), 50 (5.3%), and 65 (6.9%) received OS, tamoxifen + OS and AI + OS, respectively. No clinicopathologic features predicted OS use apart from age; patients <35 years were more likely to receive OS compared with those ≥35 years (odds ratio 2.33, p < 0.001).
Conclusions: This is the first real-world study evaluating ET treatment patterns in HR+/HER2+ premenopausal BC. OS use was uncommon and the majority received tamoxifen as the preferred ET regardless of most clinicopathologic risk factors. Additional research is needed to optimize ET decisions in young women with this distinct BC subtype.
Keywords: HER2‐positive; adjuvant therapy; breast cancer; endocrine therapy; hormone receptor positive; premenopausal.
© 2024 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Jasmine Sukumar, Andy Ni, Kai Johnson, Dionisia Quiroga, Bhuvana Ramaswamy, Robert Wesolowski, Daniel Stover, Mathew Cherian, Margaret Gatti‐Mays, Ashley Pariser, Preeti Sudheendra, and Nicole Williams have no competing interests to declare. Mridula George reports research funding from Incyte and Oncolytics. Maryam Lustberg reports consulting fees from AstraZeneca, Lily, Pfizer, Gilead, and Novartis. Sagar Sardesai reports consulting fees and/or research funding from Gilead, Stemline, and AstraZeneca.
Figures
References
-
- Schettini F, Buono G, Cardalesi C, Desideri I, De Placido S, Del Mastro L. Hormone receptor/human epidermal growth factor receptor 2‐positive breast cancer: where we are now and where we are going. Cancer Treat Rev. 2016;46:20‐26. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous