Treatments for intractable constipation in childhood
- PMID: 38895907
- PMCID: PMC11190639
- DOI: 10.1002/14651858.CD014580.pub2
Treatments for intractable constipation in childhood
Abstract
Background: Constipation that is prolonged and does not resolve with conventional therapeutic measures is called intractable constipation. The treatment of intractable constipation is challenging, involving pharmacological or non-pharmacological therapies, as well as surgical approaches. Unresolved constipation can negatively impact quality of life, with additional implications for health systems. Consequently, there is an urgent need to identify treatments that are efficacious and safe.
Objectives: To evaluate the efficacy and safety of treatments used for intractable constipation in children.
Search methods: We searched CENTRAL, MEDLINE, Embase, and two trials registers up to 23 June 2023. We also searched reference lists of included studies for relevant studies.
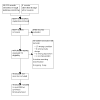
Selection criteria: We included randomised controlled trials (RCTs) comparing any pharmacological, non-pharmacological, or surgical treatment to placebo or another active comparator, in participants aged between 0 and 18 years with functional constipation who had not responded to conventional medical therapy.
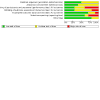
Data collection and analysis: We used standard Cochrane methods. Our primary outcomes were symptom resolution, frequency of defecation, treatment success, and adverse events; secondary outcomes were stool consistency, painful defecation, quality of life, faecal incontinence frequency, abdominal pain, hospital admission for disimpaction, and school absence. We used GRADE to assess the certainty of evidence for each primary outcome.
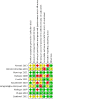
Main results: This review included 10 RCTs with 1278 children who had intractable constipation. We assessed one study as at low risk of bias across all domains. There were serious concerns about risk of bias in six studies. One study compared the injection of 160 units botulinum toxin A (n = 44) to unspecified oral stool softeners (n = 44). We are very uncertain whether botulinum toxin A injection improves treatment success (risk ratio (RR) 37.00, 95% confidence interval (CI) 5.31 to 257.94; very low certainty evidence, downgraded due to serious concerns with risk of bias and imprecision). Frequency of defecation was reported only for the botulinum toxin A injection group (mean interval of 2.6 days). The study reported no data for the other primary outcomes. One study compared erythromycin estolate (n = 6) to placebo (n = 8). The only primary outcome reported was adverse events, which were 0 in both groups. The evidence is of very low certainty due to concerns with risk of bias and serious imprecision. One study compared 12 or 24 μg oral lubiprostone (n = 404) twice a day to placebo (n = 202) over 12 weeks. There may be little to no difference in treatment success (RR 1.29, 95% CI 0.87 to 1.92; low certainty evidence). We also found that lubiprostone probably results in little to no difference in adverse events (RR 1.05, 95% CI 0.91 to 1.21; moderate certainty evidence). The study reported no data for the other primary outcomes. One study compared three-weekly rectal sodium dioctyl sulfosuccinate and sorbitol enemas (n = 51) to 0.5 g/kg/day polyethylene glycol laxatives (n = 51) over a 52-week period. We are very uncertain whether rectal sodium dioctyl sulfosuccinate and sorbitol enemas improve treatment success (RR 1.33, 95% CI 0.83 to 2.14; very low certainty evidence, downgraded due to serious concerns with risk of bias and imprecision). Results of defecation frequency per week was reported only as modelled means using a linear mixed model. The study reported no data for the other primary outcomes. One study compared biofeedback therapy (n = 12) to no intervention (n = 12). We are very uncertain whether biofeedback therapy improves symptom resolution (RR 2.50, 95% CI 1.08 to 5.79; very low certainty evidence, downgraded due to serious concerns with risk of bias and imprecision). The study reported no data for the other primary outcomes. One study compared 20 minutes of intrarectal electromotive botulinum toxin A using 2800 Hz frequency and botulinum toxin A dose 10 international units/kg (n = 30) to 10 international units/kg botulinum toxin A injection (n = 30). We are very uncertain whether intrarectal electromotive botulinum toxin A improves symptom resolution (RR 0.96, 95% CI 0.76 to 1.22; very low certainty evidence) or if it increases the frequency of defecation (mean difference (MD) 0.00, 95% CI -1.87 to 1.87; very low certainty evidence). We are also very uncertain whether intrarectal electromotive botulinum toxin A has an improved safety profile (RR 0.20, 95% CI 0.01 to 4.00; very low certainty evidence). The evidence for these results is of very low certainty due to serious concerns with risk of bias and imprecision. The study did not report data on treatment success. One study compared the injection of 60 units botulinum toxin A (n = 21) to myectomy of the internal anal sphincter (n = 21). We are very uncertain whether botulinum toxin A injection improves treatment success (RR 1.00, 95% CI 0.75 to 1.34; very low certainty evidence). No adverse events were recorded. The study reported no data for the other primary outcomes. One study compared 0.04 mg/kg oral prucalopride (n = 107) once daily to placebo (n = 108) over eight weeks. Oral prucalopride probably results in little or no difference in defecation frequency (MD 0.50, 95% CI -0.06 to 1.06; moderate certainty evidence); treatment success (RR 0.96, 95% CI 0.53 to 1.72; moderate certainty evidence); and adverse events (RR 1.15, 95% CI 0.94 to 1.39; moderate certainty evidence). The study did not report data on symptom resolution. One study compared transcutaneous electrical stimulation to sham stimulation, and another study compared dietitian-prescribed Mediterranean diet with written instructions versus written instructions. These studies did not report any of our predefined primary outcomes.
Authors' conclusions: We identified low to moderate certainty evidence that oral lubiprostone may result in little to no difference in treatment success and adverse events compared to placebo. Based on moderate certainty evidence, there is probably little or no difference between oral prucalopride and placebo in defecation frequency, treatment success, or adverse events. For all other comparisons, the certainty of the evidence for our predefined primary outcomes is very low due to serious concerns with study limitations and imprecision. Consequently, no robust conclusions could be drawn.
Copyright © 2024 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
MG: no relevant interests; published multiple Cochrane reviews relevant to the interventions in this review; NHS consultant; Co‐ordinating Editor for Cochrane Gut but was not involved in the editorial process or decision‐making for this review.
CGC: none known.
SR: no relevant interests; Professor of Paediatrics, Faculty of Medicine, University of Colombo, Sri Lanka.
MB: no relevant interests; Paediatric Gastroenterologist in an academic medical hospital.
VS: none known.
AA: no relevant interests; Physician, Sidra Medicine; Co‐ordinating Editor for Cochrane Gut but was not involved in the editorial process or decision‐making for this review.
Figures

































Update of
- doi: 10.1002/14651858.CD014580
Similar articles
-
Biofeedback for treatment of chronic idiopathic constipation in adults.Cochrane Database Syst Rev. 2014 Mar 26;2014(3):CD008486. doi: 10.1002/14651858.CD008486.pub2. Cochrane Database Syst Rev. 2014. PMID: 24668156 Free PMC article.
-
Probiotics for treatment of chronic constipation in children.Cochrane Database Syst Rev. 2022 Mar 29;3(3):CD014257. doi: 10.1002/14651858.CD014257.pub2. Cochrane Database Syst Rev. 2022. PMID: 35349168 Free PMC article.
-
Treatment for women with postpartum iron deficiency anaemia.Cochrane Database Syst Rev. 2024 Dec 13;12(12):CD010861. doi: 10.1002/14651858.CD010861.pub3. Cochrane Database Syst Rev. 2024. PMID: 39670550
-
Probiotics for management of functional abdominal pain disorders in children.Cochrane Database Syst Rev. 2023 Feb 17;2(2):CD012849. doi: 10.1002/14651858.CD012849.pub2. Cochrane Database Syst Rev. 2023. PMID: 36799531 Free PMC article.
-
Interventions for the management of abdominal pain in Crohn's disease and inflammatory bowel disease.Cochrane Database Syst Rev. 2021 Nov 29;11(11):CD013531. doi: 10.1002/14651858.CD013531.pub2. Cochrane Database Syst Rev. 2021. PMID: 34844288 Free PMC article.
Cited by
-
Towards a definition of refractory/therapy-resistant/intractable constipation in children: a cross-sectional, questionnaire-based, online survey.BMJ Paediatr Open. 2024 Dec 12;8(1):e003063. doi: 10.1136/bmjpo-2024-003063. BMJ Paediatr Open. 2024. PMID: 39667952 Free PMC article.
-
Current Treatment Options for Children with Functional Constipation-What Is in the Pipeline?Children (Basel). 2025 Jun 28;12(7):857. doi: 10.3390/children12070857. Children (Basel). 2025. PMID: 40723050 Free PMC article. Review.
References
References to studies included in this review
Ahmadi 2013 {published data only}
Bellomo‐Brandão 2003 {published data only}
Benninga 2021 {published data only}
-
- Benninga MA, Hussain SZ, Sood MR, Nurko S, Hyman P, Clifford RA, et al. Lubiprostone for pediatric functional constipation: randomized, controlled, double-blind study with long-term extension. Clinical Gastroenterology and Hepatology 2021;20(3):602-10. [DOI: 10.1016/j.cgh.2021.04.005] [PMID: ] - DOI - PubMed
-
- NCT02042183. Lubiprostone for children with constipation [A multicentre, long-term safety, efficacy, and pharmacokinetics study of lubiprostone in paediatric subjects aged ≥ 6 years to < 18 years with functional constipation]. clinicaltrials.gov/ct2/show/NCT02138136 (first received 14 May 2014).
Bongers 2009 {published data only}
-
- Bongers MEJ, den Berg MM, Reitsma JB, Voskuijl WP, Benninga MA. A randomized controlled trial of enemas in combination with oral laxative therapy for children with chronic constipation. Clinical Gastroenterology and Hepatology 2009;7(10):1069-74. [DOI: 10.1016/j.cgh.2009.06.018] [PMID: ] - DOI - PubMed
-
- ISRCTN99089299. The effect of additional use of enemas versus the standard treatment of chronic constipation in children. www.isrctn.com/ISRCTN99089299 (first received 04 August 2005).
Castilla 2021 {published data only}
-
- Castilla LKS, Serrano CAM, Figueroa AMC. Biofeedback therapy in children with chronic functional constipation and sensitivity disorders according to London criteria. Journal of Pediatric Gastroenterology and Nutrition 2021;72(Supplement 1):603.
Kajbafzadeh 2020 {published data only}
-
- IRCT20111229008554N4. Intrarectal electromotive botulinum toxin type A administration in children with refractory constipation. https://www.irct.ir/trial/28186 (first received 5 May 2018).
-
- Kajbafzadeh AM, Sharifi-Rad L, Nabavizadeh B, Ladi-Seyedian SS, Alijani M, Farahmand F, et al. Intrarectal electromotive botulinum toxin type A administration in children with intractable constipation: a randomized clinical trial. American Journal of Gastroenterology 2020;115(12):2060-7. [DOI: 10.14309/ajg.0000000000000940] [PMID: ] - DOI - PubMed
Keragiozoglou‐Lampoudi 2012 {published data only}
-
- Karagiozoglou-Lampoudi T, Daskalou E, Agakidis C, Savvidou A, Apostolou A, Vlahavas G. Personalized diet management can optimise compliance to a high-fiber, high-water diet in children with refractory functional constipation. Journal of the Academy of Nutrition and Dietetics 2012;112(5):725-9. [DOI: 10.1016/j.jand.2012.01.021] [PMID: ] - DOI - PubMed
Keshtgar 2007 {published data only}
Mugie 2014 {published data only}
-
- NCT01330381. Prucalopride in pediatric subjects with functional constipation [Trial consisting of an 8-week double-blind placebo-controlled part to evaluate efficacy, safety, tolerability and pharmacokinetics of prucalopride in paediatric subjects with functional constipation, aged ≥6 months to <18 years, followed by a 16-week open-label comparator (PEG) controlled part, to document safety and tolerability up to 24 weeks]. clinicaltrials.gov/ct2/show/NCT01330381 (first received 6 April 2011).
Southwell 2012 {published and unpublished data}
-
- ACTRN12610000418077. Children with slow transit constipation treated with transcutaneous electrical stimulation using interferrential current across the abdomen at T9-L2 compared to sham electrical stimulation - change in defecation, soiling, colonic transit and colonic activity. [TICTOC 1 - Transcutaneous interferrential current to overcome constipation- 1 phyiostherapist clinic based]. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=335524&... (first received 25 May 2010).
-
- Clarke MCC, Chase JW, Gibb S, Robertson VJ, Cato-Smith A, Hutson JM, et al. Decreased colonic transit time after transcutaneous interferential electrical stimulation in children with slow transit constipation. Journal of Pediatric Surgery 2009;44(2):408-12. [DOI: 10.1016/j.jpedsurg.2008.10.100] [PMID: ] - DOI - PubMed
-
- Southwell B. Transabdominal electrical stimulation to treat slow transit constipation in children. Neuromodulation 2012;15:63.
References to studies excluded from this review
Bekkali 2012 {published data only}
-
- Bekkali N, Liem O, Bongers MEJ, Van Wijk MP, Zegers B, Pelleboer R, et al. One year treatment of childhood constipation comparing PEG 3350 with electrolytes versus PEG 4000: a double-blind randomized controlled trial. Gastroenterology 2012;142(5):S821-22. [DOI: 10.1016/S0016-5085(12)63192-8] [PMID: ] - DOI
Broide 2001 {published data only}
Burnett 2004 {published data only}
Clarke 2012 {published data only}
-
- Clarke MCC, Catto-Smith AG, King SK, Dinning PG, Cook IJ, Chase JW, et al. Transabdominal electrical stimulation increases colonic propagating pressure waves in paediatric slow transit constipation. Journal of Pediatric Surgery 2012;47(12):2279-84. [DOI: 10.1016/j.jpedsurg.2012.09.021] [PMID: ] - DOI - PubMed
Dehghani 2012 {published data only}
Díaz 2020 {published data only}
Di Lorenzo 2020 {published data only}
-
- NCT02559817. A safety and efficacy study of a range of linaclotide doses administered orally to children ages 7-17 years, with irritable bowel syndrome with constipation (LIN-MD-63) [A multicenter, randomized, double-blind, placebo-controlled, parallel-group, safety and efficacy study of a range of linaclotide doses administered orally to children, ages 6 to 17 years, a multicenter, randomized, double-blind, placebo-controlled safety and efficacy study of a range of linaclotide doses administered orally to children ages 7-17 years, with irritable bowel syndrome with constipation (IBS-C) (ie, fulfil Rome III criteria for child/adolescent IBS and fulfil modified Rome III Criteria for child/adolescent functional constipation]. clinicaltrials.gov/ct2/show/NCT02559817 (first received 24 September 2015).
Gondo 2020 {published data only}
Gremse 2000 {published data only}
-
- Gremse D, Hixon J. Comparison of polyethylene glycol 3350, NF powder and lactulose for treatment of chronic constipation in children. Journal of Pediatric Gastroenterology and Nutrition 2000;31:S131. - PubMed
Heemskerk 2021 {published data only}
-
- Heemskerk S, Dirksen C, Van Kuijk S, Benninga M, Baeten C, Masclee A, et al. No.2-Trial: A multicenter, open-label, pragmatic randomized controlled trial on sacral neuromodulation versus personalized conservative treatment in slow-transit constipation. Colorectal Disease 2021;23(Supplement 2):4. - PMC - PubMed
-
- Heemskerk SCM, Rotteveel AH, Benninga MA, Baeten CIM, Masclee AAM, Melenhorst J, et al. Sacral neuromodulation versus personalized conservative treatment in patients with idiopathic slow-transit constipation: study protocol of the No.2-trial, a multicenter open-label randomized controlled trial and cost-effectiveness analysis. International Journal of Colorectal Disease 2018;33(4):493-501. [DOI: 10.1007/s00384-018-2978-x] [PMID: ] - DOI - PMC - PubMed
Ismail 2009 {published data only}
-
- Ismail KA, Chase J, Gibb S, Clarke M, Catto-Smith AG, Robertson VJ, et al. Daily transabdominal electrical stimulation at home increased defecation in children with slow-transit constipation: a pilot study. Journal of Pediatric Surgery 2009;44(12):2388-92. [DOI: 10.1016/j.jpedsurg.2009.07.063] [PMID: ] - DOI - PubMed
ISRCTN24521269 {published data only}
-
- ISRCTN24521269. Randomised controlled trial of injection of botulinum toxin into the internal anal sphincter versus control in treatment of chronic idiopathic constipation in children. www.isrctn.com/ISRCTN24521269 (first received 30 September 2005). [DOI: 10.1186/ISRCTN24521269] - DOI
Keshtgar 2005 {published data only}
Khan 2020 {published data only}
Loening‐Baucke 2006 {published data only}
Modin 2018 {published data only}
Nakajima 2019 {published data only}
-
- Nakajima A, Shinbo K, Ota A, Kinoshita Y. Polyethylene glycol 3350 plus electrolytes for chronic constipation: a randomized, double-blind, placebo-controlled medium-term study with an extensional 52 weeks open-label, long-term study. Journal of Gastroenterology 2019;54(9):792-803. [DOI: 10.1007/s00535-019-01581-x] [PMID: ] - DOI - PMC - PubMed
NCT03054805 {published data only}
-
- NCT03054805. The effect of probiotics on constipation, and intestinal microflora in children with functional constipation. www.clinicaltrials.gov/ct2/show/NCT03054805 (first received 14 August 2019).
Nurko 2000 {published data only}
Peng 2006 {published data only}
Radwan 2021 {published data only}
Strisciuglio 2021 {published data only}
-
- Strisciuglio C, Coppola V, Russo M, Tolone C, Marseglia GL, Verrotti A, et al. Promelaxin microenemas are non-inferior to oral polyethylene glycol for the treatment of functional constipation in young children: a randomized clinical trial. Frontiers in Pediatrics 2021;9:753938. [DOI: 10.3389/fped.2021.753938] [PMID: ] - DOI - PMC - PubMed
Thomson 2008 {published data only}
-
- Thomson MA, Jenkins HR, Bisset WM, Heuschkel R, Kalra DS, Green MR, et al. Polyethylene glycol 3350 plus electrolytes for chronic constipation in children: a double blind, placebo controlled, crossover study. Archives of Disease in Childhood 2007;92(11):996-1000. [DOI: 10.1136/adc.2006.115493] [PMID: ] - DOI - PMC - PubMed
Velasco‐Benitez 2023 {published data only}
References to studies awaiting assessment
ACTRN12620000131954 {published data only}
-
- ACTRN12620000131954. Tranabdominal electric stimulation in the treatment of chronic constipation in children - TESCCO trial [Double blind randomised, placebo-controlled clinical trial of transcutaneous electric stimulation in the treatment of chronic constipation in children]. https://anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12620000... (first received 17 December 2019).
NCT05035784 {published data only}
-
- NCT05035784. RCE With FMT in the treatment of childhood constipation [Retrograde colonic enema with fecal microbiota transplantation vs retrograde colonic enema only in the treatment of childhood constipation]. clinicaltrials.gov/ct2/show/NCT05035784 (first received 5 September 2021).
References to ongoing studies
NCT05059756 {published data only}
-
- NCT05059756. PTNS and PFR in the treatment of childhood constipation [Percutaneous tibial nerve stimulation and pelvic floor rehabilitation in the treatment of childhood constipation]. clinicaltrials.gov/ct2/show/NCT05059756 (first received 28 September 2021).
Additional references
Aali 2021
Campbell 2020
Chan 2016
Covidence [Computer program]
-
- Covidence. Version accessed 09 April 2022. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Deeks 2021
-
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Egger 1997
Emmanuel 2010
Emmett 2015
Gordon 2016
Gordon 2016a
-
- Gordon M, Wallace C, Stone J, Thomas A, Akobeng A. Probiotics for treatment of chronic constipation in children: a Cochrane systematic review. British Paediatric Allergy Immunology and Infection and British Society of Paediatric Gastroenterology, Hepatology and Nutrition 2016;101:A25.
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 31 May 2022. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Guyatt 2013
Higgins 2011
-
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.2.
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Higgins 2023
-
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook.
Hyams 2016
Irani 2008
Koppen 2018
Koppen 2018a
-
- Koppen IJN, Saps M, Lavigne JV, Nurko S, Taminiau JA, Di Lorenzo C, et al. Recommendations for pharmacological clinical trials in children with functional constipation: the Rome foundation pediatric subcommittee on clinical trials. Neurogastroenterology and Motility 2018;30(4):e12394. [DOI: 10.1111/nmo.13294] [PMID: ] - DOI - PubMed
Lefebvre 2021
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Lewis 1997
Li 2021
-
- Li T, Higgins JP, Deeks JJ, editor(s). Chapter 5: Collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
McClung 2004
-
- McClung HJ, Potter C. Rational use of laxatives in children. Advanced Pediatrics 2004;51:231-62. [PMID: ] - PubMed
Ng 2016
NICE Guideline 2013
-
- National Institute for Health and Care Excellence (NICE). Constipation in children and young people. https://www.nice.org.uk/guidance/qs62/documents/constipation-in-children... 2013. - PubMed
Philichi 2018
Portalatin 2012
Rachel 2020
-
- Rachel H, Griffith AF, Teague WJ, Hutson JM, Gibb S, Goldfeld S, et al. Polyethylene glycol dosing for constipation in children younger than 24 months: a systematic review. Journal of Pediatric Gastroenterology and Nutrition 2020;71(2):171-5. [DOI: 10.1097/MPG.0000000000002786] [PMID: ] - DOI - PubMed
Rajindrajith 2016
RevMan 2024 [Computer program]
-
- Review Manager (RevMan). Version 7.2.0. The Cochrane Collaboration, 2024. Available at revman.cochrane.org.
Shah 2011
-
- Shah ND, Chitkara D, Branda ME, Van Tilburg MA, Whitehead WE, Katusic SK, et al. Direct medical costs of constipation from childhood to early adulthood: a population-based birth cohort study. Journal of Pediatric Gastroenterology and Nutrition 2011;52(1):47-54. [DOI: 10.1097/MPG.0b013e3181e67058] [PMID: ] - DOI - PMC - PubMed
Siddiqui 2014
Siminas 2015
Sluka 2003
Southwell 2020
Tabbers 2014
-
- Tabbers MM, DiLorenzo C, Berger MY, Faure C, Langendam MW, Nurko S, et al. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. Journal of Pediatric Gastroenterology and Nutrition 2014;58(2):258-74. [DOI: 10.1097/MPG.0000000000000266] [PMID: ] - DOI - PubMed
Vriesman 2019
Vriesman 2020
-
- Vriesman MH, Wang L, Park C, Diefenbach KA, Levitt MA, Wood RJ, et al. Comparison of antegrade continence enema treatment and sacral nerve stimulation for children with severe functional constipation and fecal incontinence. Neurogastroenterology and Motility 2020;32(8):e13809. [DOI: 10.1111/nmo.13809] [PMID: ] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

