Age-dependent heat shock hormesis to HSF-1 deficiency suggests a compensatory mechanism mediated by the unfolded protein response and innate immunity in young Caenorhabditis elegans
- PMID: 38895933
- PMCID: PMC11464127
- DOI: 10.1111/acel.14246
Age-dependent heat shock hormesis to HSF-1 deficiency suggests a compensatory mechanism mediated by the unfolded protein response and innate immunity in young Caenorhabditis elegans
Abstract
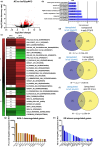
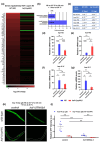
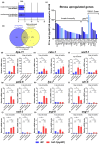
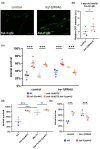
The transcription factor HSF-1 (heat shock factor 1) acts as a master regulator of heat shock response in eukaryotic cells to maintain cellular proteostasis. The protein has a protective role in preventing cells from undergoing ageing, and neurodegeneration, and also mediates tumorigenesis. Thus, modulating HSF-1 activity in humans has a promising therapeutic potential for treating these pathologies. Loss of HSF-1 function is usually associated with impaired stress tolerance. Contrary to this conventional knowledge, we show here that inactivation of HSF-1 in the nematode Caenorhabditis elegans results in increased thermotolerance at young adult stages, whereas HSF-1 deficiency in animals passing early adult stages indeed leads to decreased thermotolerance, as compared to wild-type. Furthermore, a gene expression analysis supports that in young adults, distinct cellular stress response and immunity-related signaling pathways become induced upon HSF-1 deficiency. We also demonstrate that increased tolerance to proteotoxic stress in HSF-1-depleted young worms requires the activity of the unfolded protein response of the endoplasmic reticulum and the SKN-1/Nrf2-mediated oxidative stress response pathway, as well as an innate immunity-related pathway, suggesting a mutual compensatory interaction between HSF-1 and these conserved stress response systems. A similar compensatory molecular network is likely to also operate in higher animal taxa, raising the possibility of an unexpected outcome when HSF-1 activity is manipulated in humans.
Keywords: C. Elegans; skn‐1; autophagy; cellular stress response; heat shock factor 1; heat shock proteins; heat shock response; hormesis; innate immunity; insulin‐like signaling pathway; intracellular pathogen response; proteostasis; thermotolerance; unfolded protein response.
© 2024 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Afgan, E. , Baker, D. , Batut, B. , van den Beek, M. , Bouvier, D. , Cech, M. , Chilton, J. , Clements, D. , Coraor, N. , Grüning, B. A. , Guerler, A. , Hillman‐Jackson, J. , Hiltemann, S. , Jalili, V. , Rasche, H. , Soranzo, N. , Goecks, J. , Taylor, J. , Nekrutenko, A. , & Blankenberg, D. (2018). The galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Research, 46, W537–W544. - PMC - PubMed
-
- Aman, Y. , Schmauck‐Medina, T. , Hansen, M. , Morimoto, R. I. , Simon, A. K. , Bjedov, I. , Palikaras, K. , Simonsen, A. , Johansen, T. , Tavernarakis, N. , Rubinsztein, D. C. , Partridge, L. , Kroemer, G. , Labbadia, J. , & Fang, E. F. (2021). Autophagy in healthy aging and disease. Nature Aging, 1, 634–650. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- ÚNKP-23-3/New National Excellence Program of the Ministry for Culture and Innovation
- 01062/Eötvös Loránd Research Network
- P40 OD010440/OD/NIH HHS/United States
- DKOP-23/New National Excellence Program of the Ministry for Culture and Innovation
- K132439/National Research, Development and Innovation Office
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

