Prebiotic synthesis of dihydrouridine by photoreduction of uridine in formamide
- PMID: 38896044
- PMCID: PMC11223185
- DOI: 10.1039/d4cc01823k
Prebiotic synthesis of dihydrouridine by photoreduction of uridine in formamide
Abstract
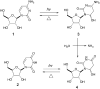
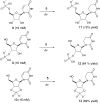
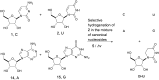
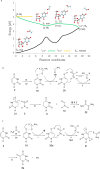
In this report, we show that a very common modification (especially in tRNA), dihydrouridine, was efficiently produced by photoreduction of the canonical pyrimidine ribonucleoside, uridine in formamide. Formamide not only acts as a solvent in this reaction, but also as the reductant. The other three components of the canonical alphabet (C, A, G) remained intact under the same conditions, suggesting that dihydrouridine might have coexisted with all four canonical RNA nucleosides (C, U, A, G) at the dawn of life.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

