A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled 14-Week Study of Mirogabalin in Chinese Patients with Diabetic Peripheral Neuropathic Pain
- PMID: 38896199
- PMCID: PMC11255142
- DOI: 10.1007/s40122-024-00617-2
A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled 14-Week Study of Mirogabalin in Chinese Patients with Diabetic Peripheral Neuropathic Pain
Abstract
Introduction: There is no approved effective drug for diabetic peripheral neuropathic pain (DPNP) in China. Gabapentinoids including mirogabalin have shown promise, although data in Chinese patients are scarce.
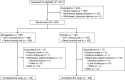
Methods: This phase 3, multicenter, randomized, double-blind, placebo-controlled trial investigated the efficacy and safety of mirogabalin for treating DPNP in China. Mirogabalin was administered at 5 mg twice daily for the first week and uptitrated to 15 mg twice daily for a total duration of 14 weeks. The primary efficacy endpoint was the change from baseline in weekly average daily pain score (ADPS) at week 14; secondary endpoints included the ADPS responder rate, Short-Form McGill Pain Questionnaire visual analogue scale score, patient global impression of change (PGIC), average daily sleep interference score (ADSIS), EuroQol 5-dimensions 5-levels (EQ-5D-5L), and incidence of treatment-emergent adverse events (TEAEs).
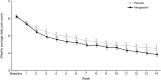
Results: Of 393 patients (mirogabalin, n = 196; placebo n = 197), the mean age was 58.2 years (mirogabalin, 58.7 years; placebo, 57.7 years) and 54.2% were male (mirogabalin, 56.1%; placebo, 52.3%). Mirogabalin elicited a greater change from baseline in the weekly ADPS vs. placebo at week 14: least-squares mean difference (95% confidence interval) vs. placebo - 0.39 (- 0.74, - 0.04), p = 0.0301. PGIC, ADSIS, and EQ-5D-5L data reflected significantly better improvements for patients receiving mirogabalin vs. placebo. The incidence of TEAEs was 75.0% and 75.1% in the mirogabalin and placebo groups, respectively. Most TEAEs were mild or moderate, and the incidence of TEAEs leading to treatment discontinuation was 2.6% in the mirogabalin group and 1.5% in the placebo group.
Conclusions: Although the effect size of mirogabalin was reduced due to the placebo effect, mirogabalin is a safe and effective treatment option for Chinese patients with DPNP.
Trial registration: ClinicalTrials.gov identifier, NCT04094662.
Keywords: China; Diabetes complications; Mirogabalin; Neuralgia; Phase 3.
© 2024. The Author(s).
Conflict of interest statement
Xiaohui Guo, Yang Yu, Yongbo Zhang, Li Sun, Yufeng Li, and Bing Song received investigator fees from Daiichi Sankyo Co., Ltd. Masayuki Baba received lecture and consultancy fees from Daiichi Sankyo Co., Ltd. Yosuke Wasaki, Kunika Kikumori, and Emiko Murayama are employees of the study sponsor, Daiichi Sankyo Co., Ltd., and Li Hang is an employee of Daiichi Sankyo (China) Holdings Co., Ltd.
Figures

References
Associated data
LinkOut - more resources
Full Text Sources
Medical

