Comparing Survival of Perihilar Cholangiocarcinoma After R1 Resection Versus Palliative Chemotherapy for Unresected Localized Disease
- PMID: 38896226
- PMCID: PMC11413094
- DOI: 10.1245/s10434-024-15582-5
Comparing Survival of Perihilar Cholangiocarcinoma After R1 Resection Versus Palliative Chemotherapy for Unresected Localized Disease
Abstract
Background: Resection of perihilar cholangiocarcinoma (pCCA) is a complex procedure with a high risk of postoperative mortality and early disease recurrence. The objective of this study was to compare patient characteristics and overall survival (OS) between pCCA patients who underwent an R1 resection and patients with localized pCCA who received palliative systemic chemotherapy.
Methods: Patients with a diagnosis of pCCA between 1997-2021 were identified from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) registry. pCCA patients who underwent an R1 resection were compared with patients with localized pCCA (i.e., nonmetastatic) who were ineligible for surgical resection and received palliative systemic chemotherapy. The primary outcome was OS.
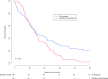
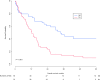
Results: Overall, 146 patients in the R1 resection group and 92 patients in the palliative chemotherapy group were included. The palliative chemotherapy group more often underwent biliary drainage (95% vs. 66%, p < 0.001) and had more vascular encasement on imaging (70% vs. 49%, p = 0.012) and CA 19.9 was more frequently >200 IU/L (64 vs. 45%, p = 0.046). Median OS was comparable between both groups (17.1 vs. 16 months, p = 0.06). Overall survival at 5 years after diagnosis was 20.0% with R1 resection and 2.2% with chemotherapy. Type of treatment (i.e., R1 resection or palliative chemotherapy) was not an independent predictor of OS (hazard ratio 0.76, 95% confidence interval 0.55-1.07).
Conclusions: Palliative systemic chemotherapy should be considered instead of resection in patients with a high risk of both R1 resection and postoperative mortality.
© 2024. The Author(s).
Conflict of interest statement
Dr. Angela Lamarca declares travel and educational support from Ipsen, Pfizer, Bayer, AAA, SirtEx, Novartis, Mylan, Delcath Advanza Pharma, and Roche; speaker honoraria from Merck, Pfizer, Ipsen, Incyte, AAA, QED, Servier, Astra Zeneca, EISAI, Roche, and Advanz Pharma; advisory and consultancy honoraria from EISAI, Nutricia Ipsen, QED, Roche, Servier, Boston Scientific, Albireo Pharma, AstraZeneca, Boehringer Ingelheim, GENFIT, TransThera Biosciences, and Taiho; principal Investigator associated Institutional Funding form QED, Merck, Boehringer Ingelheim, Servier, AstraZeneca, GenFit, Albireo Pharma; she is a member of the Knowledge Network and NETConnect Initiatives funded by Ipsen. Chiara Braconi receives honoraria from AstraZeneca (consultant, speaker, spouse employee), Incyte (consultant, speaker), Servier (consultant), Boehringer-Ingelheim (consultant); she receives research funds from Avacta, Medannex, and Servier. Jesús María Bañales declares research grants (from Incyte and Albireo), personal fees for lecturer (from Intercept, AstraZeneca and Incyte), and consulting role (for Albireo, Rubió Metabolomics, Ikan Biotech, and CYMABay). Dr. Juan Valle reports personal fees from Agios, personal fees from AstraZeneca, personal fees from Baxter, personal fees from Genoscience Pharma, personal fees from Hutchison Medipharma, personal fees from Imaging Equipment Ltd (AAA), personal fees from Incyte, personal fees from Ipsen, personal fees from Mundipharma EDO, personal fees from Mylan, grants, personal fees and non-financial support from NuCana, personal fees from QED, personal fees from Servier, personal fees from Sirtex, personal fees from Zymeworks, outside the submitted work.
Figures

References
-
- Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: epidemiology and risk factors. Liver Int. 2019;39(Suppl 1):19–31. - PubMed
-
- Popescu I, Dumitrascu T. Curative-intent surgery for hilar cholangiocarcinoma: prognostic factors for clinical decision making. Langenbecks Arch Surg. 2014;399(6):693–705. - PubMed
-
- Cho MS, Kim SH, Park SW, Lim JH, Choi GH, Park JS, et al. Surgical outcomes and predicting factors of curative resection in patients with hilar cholangiocarcinoma: 10-year single-institution experience. J Gastrointest Surg. 2012;16(9):1672–9. - PubMed
-
- Nuzzo G, Giuliante F, Ardito F, Giovannini I, Aldrighetti L, Belli G, et al. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch Surg. 2012;147(1):26–34. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

