Development of an HSV-1 production process involving serum-reduced culturing and bead-to-bead transfer
- PMID: 38896301
- PMCID: PMC11186949
- DOI: 10.1007/s00253-024-13193-4
Development of an HSV-1 production process involving serum-reduced culturing and bead-to-bead transfer
Abstract
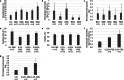
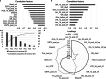
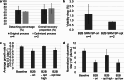
Herpes simplex virus type 1 (HSV-1) plays an important role in the field of gene therapy and viral vaccines, especially as an oncolytic virus. However, the mass production of HSV-1 viral vectors remains a challenge in the industry. In this study, a microcarrier-mediated serum-reduced medium culture was used to improve the bioprocess of HSV-1 production and increase HSV-1 yields. The composition of the culture media, which included a basal medium, serum concentration, and glutamine additive, was optimized. The process was successfully conducted in a 1 L bioreactor, and virus production was threefold greater than that of conventional processes with a 10% serum medium. The bead-to-bead transfer process was also developed to further increase scalability. In spinner flasks, the detachment rate increased from 49.4 to 80.6% when combined agitation was performed during digestion; the overall recovery proportion increased from 37.9 to 71.1% after the operational steps were optimized. Specifically, microcarrier loss was reduced during aspiration and transfer, and microcarriers and detached cells were separated with filters. Comparable cell growth was achieved with the baseline process using 2D culture as the inoculum by exchanging the subculture medium. To increase virus production after bead-to-bead transfer, critical parameters, including shear stress during digestion, TrypLE and EDTA concentrations in the subculture, and the CCI, were identified from 47 parameters via correlation analysis and principal component analysis. The optimized bead-to-bead transfer process achieved an average of 90.4% overall recovery and comparable virus production compared to that of the baseline process. This study is the first to report the optimization of HSV-1 production in Vero cells cultured on microcarriers in serum-reduced medium after bead-to-bead transfer. KEY POINTS: • An HSV-1 production process was developed that involves culturing in serum-reduced medium, and this process achieved threefold greater virus production than that of traditional processes. • An indirect bead-to-bead transfer process was developed with over 90% recovery yield in bioreactors. • HSV-1 production after bead-to-bead transfer was optimized and was comparable to that achieved with 2D culture as inoculum.
Keywords: Bead-to-bead transfer; Herpes simplex virus type 1; Microcarriers; Serum-reduced culturing; Stirred-tank bioreactor; Vero cells.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Serum-free microcarrier based production of replication deficient influenza vaccine candidate virus lacking NS1 using Vero cells.BMC Biotechnol. 2011 Aug 11;11:81. doi: 10.1186/1472-6750-11-81. BMC Biotechnol. 2011. PMID: 21835017 Free PMC article.
-
A microcarrier cell culture process for propagating rabies virus in Vero cells grown in a stirred bioreactor under fully animal component free conditions.Vaccine. 2007 May 10;25(19):3879-89. doi: 10.1016/j.vaccine.2007.01.086. Epub 2007 Feb 5. Vaccine. 2007. PMID: 17307281
-
Pseudorabies virus production using a serum-free medium in fixed-bed bioreactors with low cell inoculum density.Biotechnol Lett. 2020 Dec;42(12):2551-2560. doi: 10.1007/s10529-020-02987-x. Epub 2020 Aug 20. Biotechnol Lett. 2020. PMID: 32816175
-
Development, optimization, and scale-up of suspension Vero cell culture process for high titer production of oncolytic herpes simplex virus-1.Biotechnol J. 2024 Jan;19(1):e2300244. doi: 10.1002/biot.202300244. Epub 2023 Oct 15. Biotechnol J. 2024. PMID: 37767876
-
Vero cell upstream bioprocess development for the production of viral vectors and vaccines.Biotechnol Adv. 2020 Nov 15;44:107608. doi: 10.1016/j.biotechadv.2020.107608. Epub 2020 Aug 5. Biotechnol Adv. 2020. PMID: 32768520 Free PMC article. Review.
References
-
- Bartholomew DJ (2010) Principal components analysis. In: Peterson P, Baker E, McGaw B (eds) International Encyclopedia of Education (Third Edition). Elsevier, Oxford, pp 374–377
-
- Chen XY, Chen JY, Tong XM, Mei JG, Chen YF, Mou XZ (2020) Recent advances in the use of microcarriers for cell cultures and their ex vivo and in vivo applications. Biotechnol Lett 42. 10.1007/s10529-019-02738-7 - PubMed
-
- Chisti Y (2000) Animal-cell damage in sparged bioreactors. Trends Biotechnol 18:420–432. 10.1016/S0167-7799(00)01474-8 - PubMed
-
- Croughan MS, Hamel J-F, Wang DIC (1987) Hydrodynamic effects on animal cells grown in microcarrier cultures. Biotechnol Bioeng 29:130–141. 10.1002/bit.260290117 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

