Activation of the MAPK network provides a survival advantage during the course of COVID-19-induced sepsis: a real-world evidence analysis of a multicenter COVID-19 Sepsis Cohort
- PMID: 38896372
- PMCID: PMC11825614
- DOI: 10.1007/s15010-024-02325-7
Activation of the MAPK network provides a survival advantage during the course of COVID-19-induced sepsis: a real-world evidence analysis of a multicenter COVID-19 Sepsis Cohort
Abstract
Purpose: There is evidence that lower activity of the RAF/MEK/ERK network is associated with positive outcomes in mild and moderate courses of COVID-19. The effect of this cascade in COVID-19 sepsis is still undetermined. Therefore, we tested the hypothesis that activity of the RAF/MEK/ERK network in COVID-19-induced sepsis is associated with an impact on 30-day survival.
Methods: We used biomaterial from 81 prospectively recruited patients from the multicentric CovidDataNet.NRW-study cohort (German clinical trial registry: DRKS00026184) with their collected medical history, vital signs, laboratory parameters, microbiological findings and patient outcome. ERK activity was measured by evaluating ERK phosphorylation using a Proximity Ligation Assay.
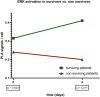
Results: An increased ERK activity at 4 days after diagnosis of COVID-19-induced sepsis was associated with a more than threefold increased chance of survival in an adjusted Cox regression model. ERK activity was independent of other confounders such as Charlson Comorbidity Index or SOFA score (HR 0.28, 95% CI 0.10-0.84, p = 0.02).
Conclusion: High activity of the RAF/MEK/ERK network during the course of COVID-19 sepsis is a protective factor and may indicate recovery of the immune system. Further studies are needed to confirm these results.
Keywords: COVID-19; ERK; MAPK; MEK; PLA; RAF; Sepsis.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Ethics Committee of the Medical Faculty of the University of Bochum (Ruhr-University Bochum, registry number 18–6606-BR, German clinical trial registry: DRKS00026184). Informed consent forms were available for all included patients or their respective legal representatives, if patients were unable to provide informed consent at the time of study inclusion. Competing interests: The authors declare that they have no competing interests. Consent for publication: Not applicable.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

