Development of a nanoparticle-based tendon-targeting drug delivery system to pharmacologically modulate tendon healing
- PMID: 38896625
- PMCID: PMC11186494
- DOI: 10.1126/sciadv.adn2332
Development of a nanoparticle-based tendon-targeting drug delivery system to pharmacologically modulate tendon healing
Abstract
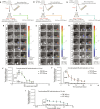
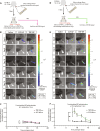
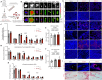
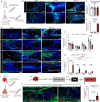
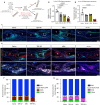
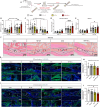
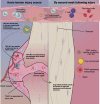
Satisfactory healing following acute tendon injury is marred by fibrosis. Despite the high frequency of tendon injuries and poor outcomes, there are no pharmacological therapies in use to enhance the healing process. Moreover, systemic treatments demonstrate poor tendon homing, limiting the beneficial effects of potential tendon therapeutics. To address this unmet need, we leveraged our existing tendon healing spatial transcriptomics dataset and identified an area enriched for expression of Acp5 (TRAP) and subsequently demonstrated robust TRAP activity in the healing tendon. This unexpected finding allowed us to refine and apply our existing TRAP binding peptide (TBP) functionalized nanoparticle (NP) drug delivery system (DDS) to facilitate improved delivery of systemic treatments to the healing tendon. To demonstrate the translational potential of this DDS, we delivered niclosamide (NEN), an S100a4 inhibitor. While systemic delivery of free NEN did not alter healing, TBP-NPNEN enhanced both functional and mechanical recovery, demonstrating the translational potential of this approach to enhance the tendon healing process.
Figures


Update of
-
Development of a Nanoparticle-Based Tendon-Targeting Drug Delivery System to Pharmacologically Modulate Tendon Healing.bioRxiv [Preprint]. 2023 Nov 30:2023.11.29.569204. doi: 10.1101/2023.11.29.569204. bioRxiv. 2023. Update in: Sci Adv. 2024 Jun 21;10(25):eadn2332. doi: 10.1126/sciadv.adn2332. PMID: 38076889 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

