Genetic identification of medullary neurons underlying congenital hypoventilation
- PMID: 38896627
- PMCID: PMC11186509
- DOI: 10.1126/sciadv.adj0720
Genetic identification of medullary neurons underlying congenital hypoventilation
Abstract
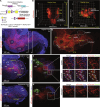
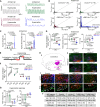
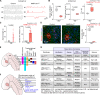
Mutations in the transcription factors encoded by PHOX2B or LBX1 correlate with congenital central hypoventilation disorders. These conditions are typically characterized by pronounced hypoventilation, central apnea, and diminished chemoreflexes, particularly to abnormally high levels of arterial PCO2. The dysfunctional neurons causing these respiratory disorders are largely unknown. Here, we show that distinct, and previously undescribed, sets of medullary neurons coexpressing both transcription factors (dB2 neurons) account for specific respiratory functions and phenotypes seen in congenital hypoventilation. By combining intersectional chemogenetics, intersectional labeling, lineage tracing, and conditional mutagenesis, we uncovered subgroups of dB2 neurons with key functions in (i) respiratory tidal volumes, (ii) the hypercarbic reflex, (iii) neonatal respiratory stability, and (iv) neonatal survival. These data provide functional evidence for the critical role of distinct medullary dB2 neurons in neonatal respiratory physiology. In summary, our work identifies distinct subgroups of dB2 neurons regulating breathing homeostasis, dysfunction of which causes respiratory phenotypes associated with congenital hypoventilation.
Figures





References
-
- Ceccherini I., Kurek K. C., Weese-Mayer D. E., Developmental disorders affecting the respiratory system: CCHS and ROHHAD. Handb. Clin. Neurol. 189, 53–91 (2022). - PubMed
-
- Weese-Mayer D. E., Berry-Kravis E. M., Ceccherini I., Keens T. G., Loghmanee D. A., Trang H.; ATS Congenital Central Hyproventilation Syndrome Subcommittee , An official ATS clinical policy statement: Congenital central hypoventilation syndrome: Genetic basis, diagnosis, and management. Am. J. Respir. Crit. Care Med. 181, 626–644 (2010). - PubMed
-
- Weese-Mayer D. E., Berry-Kravis E. M., Ceccherini I., Rand C. M., Congenital central hypoventilation syndrome (CCHS) and sudden infant death syndrome (SIDS): Kindred disorders of autonomic regulation. Respir. Physiol. Neurobiol. 164, 38–48 (2008). - PubMed
-
- Rand C. M., Carroll M. S., Weese-Mayer D. E., Congenital central hypoventilation syndrome. Clin. Chest Med. 35, 535–545 (2014). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

