Patient-Derived Tumor Organoids Combined with Function-Associated ScRNA-Seq for Dissecting the Local Immune Response of Lung Cancer
- PMID: 38896792
- PMCID: PMC11336893
- DOI: 10.1002/advs.202400185
Patient-Derived Tumor Organoids Combined with Function-Associated ScRNA-Seq for Dissecting the Local Immune Response of Lung Cancer
Abstract
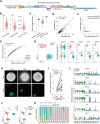
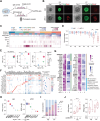
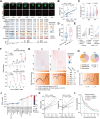
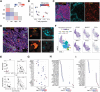
In vitro models coupled with multimodal approaches are needed to dissect the dynamic response of local tumor immune microenvironment (TIME) to immunotherapy. Here the patient-derived primary lung cancer organoids (pLCOs) are generated by isolating tumor cell clusters, including the infiltrated immune cells. A function-associated single-cell RNA sequencing (FascRNA-seq) platform allowing both phenotypic evaluation and scRNA-seq at single-organoid level is developed to dissect the TIME of individual pLCOs. The analysis of 171 individual pLCOs derived from seven patients reveals that pLCOs retain the TIME heterogeneity in the parenchyma of parental tumor tissues, providing models with identical genetic background but various TIME. Linking the scRNA-seq data of individual pLCOs with their responses to anti-PD-1 (αPD-1) immune checkpoint blockade (ICB) allows to confirm the central role of CD8+ T cells in anti-tumor immunity, to identify potential tumor-reactive T cells with a set of 10 genes, and to unravel the factors regulating T cell activity, including CD99 gene. In summary, the study constructs a joint phenotypic and transcriptomic FascRNA-seq platform to dissect the dynamic response of local TIME under ICB treatment, providing a promising approach to evaluate novel immunotherapies and to understand the underlying molecular mechanisms.
Keywords: function‐associated single‐cell RNA sequencing; immune checkpoint blockade; local tumor immune microenvironment; primary lung cancer organoid; tumor micro‐niche.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Binnewies M., Roberts E. W., Kersten K., Chan V., Fearon D. F., Merad M., Coussens L. M., Gabrilovich D. I., Ostrand‐Rosenberg S., Hedrick C. C., Vonderheide R. H., Pittet M. J., Jain R. K., Zou W., Howcroft T. K., Woodhouse E. C., Weinberg R. A., Krummel M. F., Nat. Med. 2018, 24, 541. - PMC - PubMed
-
- Joshi K., de Massy M. R., Ismail M., Reading J. L., Uddin I., Woolston A., Hatipoglu E., Oakes T., Rosenthal R., Peacock T., Ronel T., Noursadeghi M., Turati V., Furness A. J. S., Georgiou A., Wong Y. N. S., Ben Aissa A., Sunderland M. W., Jamal‐Hanjani M., Veeriah S., Birkbak N. J., Wilson G. A., Hiley C. T., Ghorani E., Guerra‐Assuncao J. A., Herrero J., Enver T., Hadrup S. R., Hackshaw A., Peggs K. S., et al., Nat. Med. 2019, 25, 1549. - PMC - PubMed
-
- Tuveson D., Clevers H., Science 2019, 364, 952. - PubMed
MeSH terms
Substances
Grants and funding
- 22127804/National Natural Science Foundation of China
- 31971325/National Natural Science Foundation of China
- RDL2023-05/Peking University People's Hospital Scientific Research Development Funds
- RDX2023-07/Peking University People's Hospital Scientific Research Development Funds
- Y-HR2020MS-0863/Beijing Xisike Clinical Oncology Research Foundation
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
