A Non-Coding Oligonucleotide Recruits Cutaneous CD11b+ Cells that Inhibit Thelper Responses and Promote Tregs
- PMID: 38896803
- PMCID: PMC11336929
- DOI: 10.1002/advs.202400260
A Non-Coding Oligonucleotide Recruits Cutaneous CD11b+ Cells that Inhibit Thelper Responses and Promote Tregs
Abstract
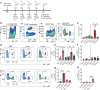
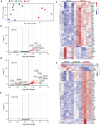
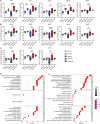
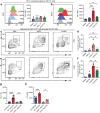
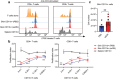
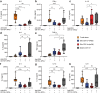
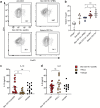
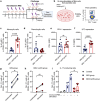
Skin-resident antigen-presenting cells (APC) play an important role in maintaining peripheral tolerance via immune checkpoint proteins and induction of T regulatory cells (Tregs). However, there is a lack of knowledge on how to expand or recruit immunoregulatory cutaneous cells without causing inflammation. Here, it is shown that administration of a non-coding single-stranded oligonucleotide (ssON) leads to CCR2-dependent accumulation of CD45+CD11b+Ly6C+ cells in the skin that express substantial levels of PD-L1 and ILT3. Transcriptomic analyses of skin biopsies reveal the upregulation of key immunosuppressive genes after ssON administration. Functionally, the cutaneous CD11b+ cells inhibit Th1/2/9 responses and promote the induction of CD4+FoxP3+ T-cells. In addition, ssON treatment of imiquimod-induced inflammation results in significantly reduced Th17 responses. It is also shown that induction of IL-10 production in the presence of cutaneous CD11b+ cells isolated after ssON administrations is partly PD-L1 dependent. Altogether, an immunomodulatory ssON is identified that can be used therapeutically to recruit cutaneous CD11b+ cells with the capacity to dampen Th cells.
Keywords: T helper cells; antigen‐presenting cells; cytokines; oligonucleotides; programmed‐death ligand 1; skin.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
A.L.S. and A.D. declare ownership in TIRmed Pharma having IPR related to ssON. K.K., E.A.R., and M.W.H. declares no conflict of interest.
Figures
Similar articles
-
Mesenteric lymph node CD11b- CD103+ PD-L1High dendritic cells highly induce regulatory T cells.Immunology. 2017 Sep;152(1):52-64. doi: 10.1111/imm.12747. Epub 2017 Jun 1. Immunology. 2017. PMID: 28423181 Free PMC article.
-
Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells.Proc Natl Acad Sci U S A. 2021 Jul 27;118(30):e2023739118. doi: 10.1073/pnas.2023739118. Proc Natl Acad Sci U S A. 2021. PMID: 34301886 Free PMC article.
-
Differential Involvement of Programmed Cell Death Ligands in Skin Immune Responses.J Invest Dermatol. 2022 Jan;142(1):145-154.e8. doi: 10.1016/j.jid.2021.06.026. Epub 2021 Jul 24. J Invest Dermatol. 2022. PMID: 34310947
-
Programmed Cell Death Ligand (PD-L)-1 Contributes to the Regulation of CD4+ T Effector and Regulatory T Cells in Cutaneous Leishmaniasis.Front Immunol. 2020 Oct 22;11:574491. doi: 10.3389/fimmu.2020.574491. eCollection 2020. Front Immunol. 2020. PMID: 33193363 Free PMC article.
-
Antigen-presenting cell production of IL-10 inhibits T-helper 1 and 17 cell responses and suppresses colitis in mice.Gastroenterology. 2011 Aug;141(2):653-62, 662.e1-4. doi: 10.1053/j.gastro.2011.04.053. Epub 2011 Apr 30. Gastroenterology. 2011. PMID: 21679711 Free PMC article.
References
-
- Ou L.‐S., Huang J.‐L., Clin. Rev. Allergy Immunol. 2007, 33, 191. - PubMed
-
- Kolkhir P., Akdis C. A., Akdis M., Bachert C., Bieber T., Canonica G. W., Guttman‐Yassky E., Metz M., Mullol J., Palomares O., Renz H., Ständer S., Zuberbier T., Maurer M., Nat. Rev. Drug Discovery 2023, 22, 743. - PubMed
-
- Leung D. Y. M., Jain N., Leo H. L., Curr. Opin. Immunol. 2003, 15, 634. - PubMed
-
- Nicolàs L. S. S., Czarnowicki T., Akdis M., Pujol R. M., Lozano‐Ojalvo D., Leung D. Y. M., Guttman‐Yassky E., Santamaria‐Babí L. F., Allergy 2023, 14, 15. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
